Abstract
Co-stimulation through members of the tumor necrosis factor receptor (TNFR) family appears to be critical for the generation of T cells with optimal effector-memory properties for adoptive cell therapy. Our work suggests that continuous 4–1BB/CD137 co-stimulation is required for the expansion of T cells with an optimal therapeutic profile and that the administration of 4–1BB agonists upon adoptive cell transfer further improves antitumor T-cell functions.
The adoptive reinfusion of autologous tumor-infiltrating lymphocytes (TILs) expanded (and optionally activated) ex vivo is emerging as an effective salvage approach for metastatic melanoma patients who have progressed in spite of several therapeutic regimens, including approaches based on the blockade of cytotoxic T lymphocyte-associated protein 4 (CTLA-4)- and programmed death 1 (PD1)-regulated immunological checkpoints.Citation1,Citation2 In this setting, TILs are first grown from tumor fragments in the presence of high interleukin-2 (IL-2) concentrations, followed by a rapid expansion protocol (REP) in which T cells are induced to rapidly proliferate by means of T-cell receptor (TCR) stimulation, IL-2 and irradiated peripheral blood mononuclear cells (operating as feeder cells).Citation1,Citation3 Upon REP, TILs are purified and adoptively transferred into patients in the context of 1–2 cycles of IL-2-based therapy, to promote their expansion in vivo. Both the quality of transferred cells, in particular with respect to their memory and effector properties, and in vivo microenvironment are key determinants of the persistence of TILs upon transfer, hence impacting on their ability to exert antineoplastic effects. The pre-conditioning of the host by means of a transiently lymphodepleting chemotherapeutic regimen facilitates TIL persistence by stimulating their homeostatic expansion and has led to considerably improved response rates.Citation2 However, the expansion of TILs, notably CD8+ cells, with optimal effector properties and persisting as a pool of memory cells that turn over and differentiate into effectors to maintain durable antitumor responses remains elusive.
Co-stimulatory signals are required for the productive activation, expansion and differentiation of naïve T cells, notably as they prevent anergy and activation-induced cell death (AICD). Thus, CD28, an immunoglobulin protein family member, and CD27, a member of the tumor necrosis factor receptor (TNFR) superfamily, deliver crucial co-stimulatory signals during the initial phases of T-cell activation.Citation4 Conversely, TNFR-like receptors such as 4–1BB/CD137 and OX40/CD134 play a prominent role as co-stimulatory molecules especially at the effector-memory stage.Citation5 Of note, 4–1BB has also been reported to stimulate the expression of CD28, thus increasing the susceptibility of T cells to co-stimulation.Citation6
For the most part, TILs consist of differentiated effector-memory cells that have lost CD27 and CD28 expression, thus 1) being susceptible to AICD and 2) being hyporesponsive to TCR stimulation.Citation7 At least in part, this reflects the upregulation of PD1 and other co-inhibitory receptors on the cell surface. We have found that CD8+ TILs also express high levels of 4–1BB (and OX40, though to a lesser extent) upon TCR activation.Citation8,Citation9 In particular, a significant frequency of freshly isolated CD8+ TILs express 4–1BB as well as PD1. As the simultaneous expression of these markers is indicative of recent antigenic stimulation, CD8+4–1BB+PD1+ TILs may be enriched of tumor-specific lymphocytes. During initial IL-2-based ex vivo expansion phase, 4–1BB is downregulated, but can be rapidly upregulated again upon TCR stimulation.Citation9 We found that CD8+ TILs subjected to the REP promptly re-express 4–1BB, allowing for further co-stimulation.Citation8
These observations prompted us to investigate the effects of 4–1BB co-stimulation on the differentiation and function of melanoma-derived CD8+TILs in the course of ex vivo expansion. Initially, we were interested in post-REP TILs in which the expression of both CD27 and CD28 is lost (). We found that post-REP TILs are highly sensitive to AICD even in the presence of nominal levels of TCR stimulation.Citation9 This might represent a dilemma in vivo, when TILs get in contact with their cognate antigen, and may explain why many T-cell clones are deleted soon upon adoptive transfer (). However, 4–1BB was rapidly upregulated by TILs subjected to TCR stimulation, and the activation of 4–1BB with either its natural ligand (4–1BB-L) or agonistic anti-4–1BB antibodies not only prevented AICD, but also resulted in continued cell expansion and improved memory and effector phenotype.Citation9 These results suggest that the provision of exogenous 4–1BB co-stimulation in vivo may be useful to improve the persistence and antitumor activity of adoptively transferred (and perhaps endogenous) T cells. Clinical trials based on 4–1BB-activating agents would be useful to test this hypothesis. Of note, we found that the 4–1BB co-stimulatory pathway can be triggered with relatively low levels of agonists. Thus, 4–1BB-activating agents may be able to induce therapeutic antitumor responses along with limited (in incidence and severity) side effects.
Figure 1. Role for 4–1BB co-stimulation during the expansion of tumor-infiltrating lymphocytes ex vivo and subsequent adoptive cell transfer. We propose that the continuative co-stimulation of tumor-infilrating lymphocytes (TILs) via 4–1BB throughout their expansion ex vivo and upon their reinfusion into patients accelerates the generation of high amounts of CD8+ T cells exhibiting an improved effector-memory profile and increased in vivo persistence. Different stages of TIL expansion—including the initial outgrowth from tumor fragments followed by the rapid expansion protocol (REP) to generate the final infusion product—are schematized. Past expansion protocols (bottom half) have not provided enough co-stimulatory signals, to TILs, in particular through members of the TNF-R family such as 4-1BB resulting in infusion products that are enriched in CD4+ T cells or are generally unable to mediate therapeutic antitumor effects due to poor effector-memory function and in vivo persistence. Conversely, TILs exposed to 4–1BB agonists (top half) maintain the expression of effector-memory markers while upregulating anti-apoptotic proteins and co-stimulatory receptors other than 4–1BB, like CD28. The infusion products generated by this protocol are enriched in CD8+ T cells, have improved survival and expansion capacity in vivo after adoptive transfer, and may exert more robust tumor control.
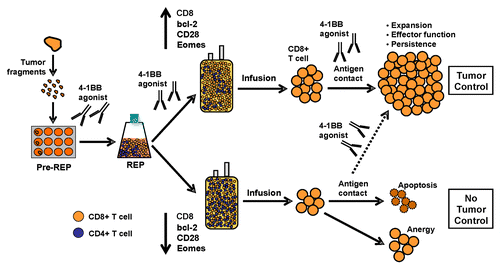
We have recently reported that the total number of TILs, as well as the total number of infused CD8+ cells, correlates with clinical responses in melanoma patients undergoing adoptive cell therapy (ACT).Citation1 However, current protocols for TIL expansion are not designed to facilitate the specific expansion of CD8+ cells, and frequently their CD4+ counterparts predominate. We found that during the first few days of the REP, CD8+ T cells express high levels of 4–1BB, prompting us to test whether 4–1BB co-stimulation would affect the expansion or overall quality of TILs, specifically within the CD8+ subset, for ACT. Importantly, the use of a fully human agonistic anti-4–1BB antibody during the REP dramatically increased the yield of CD8+ T cells. These cells maintained memory markers such as CD28 and eomesodermin while expressing increased levels of anti-apoptotic molecules, perforin and granzyme B.Citation8 4–1BB-co-stimulated TILs also exhibited an improved proliferative response to melanoma-associated antigens.Citation8 Of note, an alternative way of delivering 4–1BB co-stimulatory signals to expanding TILs is provided by so-called “artificial antigen-presenting cells,” for instance, myelogenous leukemia K562 cells engineered to overexpress 4–1BB-L or another ligand for co-stimulatory receptors.Citation10
Another shortcoming of current ACT protocols is an inefficient expansion of sufficient numbers of CD8+ T cells from initial tumor biopsies, the time that is required to initially grow out enough TILs for larger-scale rapid expansion or "REP" (), and the heterogeneous nature of expanded cells, especially in terms of antitumor reactivity. Our data showing that CD8+ TILs freshly isolated from melanoma lesions express 4–1BB have also prompted us to investigate the role of 4–1BB ligation during the “pre-REP” TIL expansion phase (). Preliminary data suggest that 4–1BB co-stimulation accelerates the outgrowth of CD8+ TILs enriched in tumor-specific cells (Pilon-Thomas and Chacon, unpublished observations).
In summary, our results suggest that provision of continuative 4–1BB co-stimulation will generate optimal T cells for ACT and to improve their biological activity upon reinfusion.
| Abbreviations: | ||
| ACT | = | adoptive cell therapy |
| AICD | = | activation-induced cell death |
| PBMC | = | peripheral blood mononuclear cell |
| REP | = | rapid expansion protocol |
| TIL | = | tumor-infiltrating lymphocyte |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Radvanyi LG, Bernatchez C, Zhang M, Fox PS, Miller P, Chacon J, et al. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin Cancer Res 2012; 18:6758 - 70; http://dx.doi.org/10.1158/1078-0432.CCR-12-1177; PMID: 23032743
- Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 2011; 17:4550 - 7; http://dx.doi.org/10.1158/1078-0432.CCR-11-0116; PMID: 21498393
- Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother 2003; 26:332 - 42; http://dx.doi.org/10.1097/00002371-200307000-00005; PMID: 12843795
- Bertram EM, Dawicki W, Sedgmen B, Bramson JL, Lynch DH, Watts TH. A switch in costimulation from CD28 to 4-1BB during primary versus secondary CD8 T cell response to influenza in vivo. J Immunol 2004; 172:981 - 8; PMID: 14707071
- Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol 2005; 23:23 - 68; http://dx.doi.org/10.1146/annurev.immunol.23.021704.115839; PMID: 15771565
- Habib-Agahi M, Jaberipour M, Searle PF. 4-1BBL costimulation retrieves CD28 expression in activated T cells. Cell Immunol 2009; 256:39 - 46; http://dx.doi.org/10.1016/j.cellimm.2009.01.003; PMID: 19217084
- Li Y, Liu S, Hernandez J, Vence L, Hwu P, Radvanyi L. MART-1-specific melanoma tumor-infiltrating lymphocytes maintaining CD28 expression have improved survival and expansion capability following antigenic restimulation in vitro. J Immunol 2010; 184:452 - 65; http://dx.doi.org/10.4049/jimmunol.0901101; PMID: 19949105
- Chacon JA, Wu RC, Sukhumalchandra P, Molldrem JJ, Sarnaik A, Pilon-Thomas S, et al. Co-stimulation through 4-1BB/CD137 improves the expansion and function of CD8(+) melanoma tumor-infiltrating lymphocytes for adoptive T-cell therapy. PLoS One 2013; 8:e60031; http://dx.doi.org/10.1371/journal.pone.0060031; PMID: 23560068
- Hernandez-Chacon JA, Li Y, Wu RC, Bernatchez C, Wang Y, Weber JS, et al. Costimulation through the CD137/4-1BB pathway protects human melanoma tumor-infiltrating lymphocytes from activation-induced cell death and enhances antitumor effector function. J Immunother 2011; 34:236 - 50; http://dx.doi.org/10.1097/CJI.0b013e318209e7ec; PMID: 21389874
- Sluijter BJ, van den Hout MF, Stam AG, Lougheed SM, Suhoski MM, van den Eertwegh AJ, et al. 4-1BB-mediated expansion affords superior detection of in vivo primed effector memory CD8+ T cells from melanoma sentinel lymph nodes. Clin Immunol 2010; 137:221 - 33; http://dx.doi.org/10.1016/j.clim.2010.07.009; PMID: 20708974