Abstract
In previous studies we demonstrated that the staphylococcal α-toxin inhibits adhesion and invasion of S. aureus by epithelial cells through binding to α5β1 integrin, a receptor of fibronectin. Moreover, we revealed that a H35A mutation abolishes the cytotoxicity of α-toxin completely. These findings led us to hypothesize that the H35A mutated α-toxin may be explored as a potential inhibitor for bacterial adhesion and invasion of epithelial cells. In this study, we examined the impact of the H35A α-toxin on staphylococcal capacity of adhering to and invading into epithelial cells and found that the addition of H35A α-toxin in the culture medium dramatically inhibited S. aureus’ ability to adhere to and internalize into epithelial cells. Importantly, we demonstrated that both the staphylococcal α-toxin and H35A mutated α-toxin are capable of retarding the adhesion and invasion of epithelial cells by Streptococcus pyogenes. These findings suggest that the H35A toxoid has the potential to be utilized as an inhibitor of S. aureus and S. pyogenes ability to adhere to and invade epithelial cells.
Introduction
Staphylococcus aureus is a commensal and typically colonizes the nares, pharynx, axillae, vagina and skin.Citation1,Citation2 This opportunistic pathogen causes a variety of diseases including endocarditis, osteomyelitis, pneumonia, toxic shock syndrome and septicemia.Citation3 Group A streptococcus is a major human pathogen and this organism is responsible for a variety of infections, including pharyngitis, impetigo and toxic shock syndrome.Citation4
Microbial adherence to host cells is a critical step to initiate infection and involves a complex process. The bacterial host-cell interactions induce cytoskeleton reshuffling in the host cell to allow for bacteria internalization and to cause further infection. S. aureus and Streptococcus pyogenes express MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) adhesins and invasins, which are involved in adhesion and/or invasion of non-phagocytic cells by specifically binding to ligands of the host extracellular matrix components, such as collagen, fibrinogen and fibronectin.Citation5-Citation9 The internalization of bacterial pathogens by host cells is modulated via integrins binding to the ECM proteins-adhesin/invasin complex, resulting in activation of host cell signal transduction pathways leading to actin-mediated “zipper phagocytosis” of adhered bacterial cells.Citation10 The adherence of S. aureus and S. pyogenes to host cell integrins leads to the activation of Src family protein-tyrosine kinase that triggers the rearrangement of host cell actin cytoskeleton, which is required for the host cell to internalize bacterial cells.Citation11,Citation12
The staphylococcal α-toxin can cause apoptosis and death of variety of host cells,Citation13,Citation14 and is an important virulence factor in experimental brain abscesses,Citation15 intraperitoneal infection modelsCitation16,Citation17 and pneumonia.Citation18 However, it has also been reported that the overproduction of α-toxin significantly reduces virulence in an experimental endocarditis,Citation19 and that small colony variants of S. aureus (SCVs) have reduced production of α-toxin and increased adhesion to human epithelial cells.Citation20 Recently, we have revealed that the overproduction of α-toxin significantly alleviates the capability of S. aureus to adhere to and be internalized into epithelial cells.Citation21 Also, we demonstrated that staphylococcal α-toxin is able to directly bind to integrin β1, a major Fn receptor on the host cell membrane, which in turn interrupts the Fn-mediated bridge formation between bacterial surface proteins and integrin β1 and leads to the elimination of bacterial adhesion and internalization by the host cells.Citation21 The interaction of α-toxin with α5β1-integrin contributes to the cytotoxicity of α-toxin that is required for S. aureus to induce apoptosis and death of the epithelial cells.Citation22 Given the fact that many bacterial pathogens produce α-toxin, a family of pore forming toxins, an α-toxin mediated inhibitory effect on the bacterial adhesion to and internalization by eukaryotic cells may exist, thus blocking the connection between the microbe and the integrins of host cells. It has been reported that the H35L mutagenesis leads to the elimination of pathogenicity of α-toxin in an animal model.Citation23 We also found that the H35L or H35A mutation in α-toxin abrogates the cytotoxicity of α-toxin in epithelial A549 cells.Citation22,Citation24
Based upon the above findings, we hypothesized that the H35A mutated α-toxin (toxoid) could be used as a potential inhibitor of bacterial adhesion and invasion of epithelial cells, which in turn may interfere with bacterial colonization. In this study, we first determined the impact of the staphylococcal α-toxin on adhesion and invasion of group A streptococci using the human lung epithelial cell line (A549). Then we examined the influence of the staphylococcal H35A mutated α-toxin on adhesion and invasion of S. aureus and group A streptococci. In addition, we examined the effect of the staphylococcal H35A mutated α-toxin on the cytotoxicity of group A streptococci.
Results and Discussion
The H35A mutated α-toxin inhibits the capacity of S. aureus to adhere to and invade into epithelial cells (A549)
In previous studies we determined the effect of the staphylococcal α-toxin on the ability of S. aureus to adhere to and internalize into epithelial cells. We have demonstrated that the overexpression of α-toxin or addition of purified α-toxin dramatically inhibits the number of S. aureus adhered to and internalized into the epithelial cells, whereas the downregulation of α-toxin increases S. aureus adhesion to and invasion of the epithelial cells.Citation22 We and other investigators have demonstrated the lack of cytotoxicity of H35L and H35A mutated α-toxin.Citation23,Citation24 We also found that the mutated H35A can interrupt pore formation and block the cytotoxicity of α-toxin. Thus, we postulated that the H35A toxoid possibly interfere with the capability of bacterial adhesion and invasion.
To examine this possibility, we purified H35A α-toxin from the overexpression S. aureus as describedCitation21 and determined its impact on bacterial adhesion and invasion by the addition of purified H35A α-toxin into the culture medium. Before S. aureus infection, the A549 monolayer cells were treated with the H35A α-toxin or control wild-type α-toxin for 15 min. Consistent with our previous findingsCitation21 the result showed that the addition of purified α-toxin remarkably decreased the number of S. aureus adhered to the cells (). Moreover, the addition of purified H35A α-toxin dramatically inhibited S. aureus adhesion to the epithelial cells () and internalization by the epithelial cells (). In contrast, the purified culture supernatant proteins from the control strain did not affect bacterial adhesion and invasion (data not shown).
Figure 1. (A) MRSA WCUH29 adherence to A549 cells. (B) Intracellular invasion. Relative adherence and relative invasion were calculated as described in experimental procedures. Data are the means ± standard errors of the means from three infected monolayer wells. CT, control WCUH29 only. The symbol * represents p < 0.05 between control and treatment group.
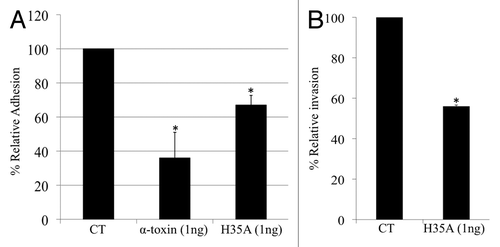
In addition, it was observed that the H35A mutation in α-toxin also reduced the inhibitory effect of α-toxin on S. aureus adhesion to and invasion of the epithelial cells (). The possible explanation is that the H35A mutation may affect the toxoid’s ability to bind with α5β1-integrin, the receptor of fibronectin (Fn), as we have revealed that α-toxin binds to α5β1-integrin and interrupts bacterial adhesion and invasion.
To examine the possibility that the elevated capacity of bacterial adhesion and invasion may result from the cytotoxic effect of α-toxin, we determined the impact of the addition of purified α-toxin in the cell culture medium on the viability and morphology of epithelial cells during the period of adhesion and invasion assays. We found that exposing the epithelial cells to sublethal dose of wild-type α-toxin or H35A mutated α-toxin for a short period of time (2 h) had no remarkable effect on their morphology under the microscope observations and viability based upon the cytotoxicity assays (data not shown), indicating that the elevated adhesion is not due to the cytotoxic effects of the wild-type α-toxin. These findings suggest that it is possible to utilize H35A mutated α-toxin (toxoid) as an inhibitor of S. aureus adhesion to and internalization by epithelial cells, which may eliminate bacterial pathogenicity.
The H35A mutated α-toxin inhibits group A streptococci adhesion to and invasion of epithelial cells (A549)
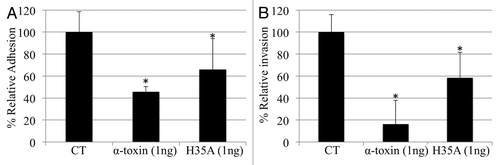
Streptococci can utilize a similar signaling pathway as S. aureus to adhere to and invade into epithelial cells via the host cell matrix protein fibrionectin (Fn) binding to the α5β1-integrin receptor on epithelial cells and the cell wall-associated Fn-binding proteins of group A streptococci.Citation10-Citation12 This led us to test the possibility that the staphylococcal α-toxin and/or H35A mutated α-toxin may also interrupt the adhesion and invasion of epithelial cells by group A streptococci. We found that the addition of purified staphylococcal α-toxin in the culture medium dramatically alleviated the competence of group A streptococci adhesion to and internalization by the A549 epithelial cells (). Furthermore, we found that the H35A mutated α-toxin was able to significantly reduce the capacity of S. pyogenes to adhere to and internalize into the epithelial cells (). The above data collectively demonstrated that the staphylococcal H35A α-toxin is able to interfere with S. pyogenes internalization by human epithelial cells. The inhibitory effect of H35A mutated α-toxin on the capacity of S. pyogenes to adhere to and invade into the epithelial cells may function through the interruption of α5β1-integrin signaling pathway, as streptococci utilize the α5β1-integrin as a major receptor to internalize epithelial cells via the cell wall-associated Fn-binding proteins of group A streptococci.Citation10-Citation12 The above findings suggest that the H35A mutated α-toxin may be utilized as an antibacterial agent against the streptococcal adhesion and invasion of host cells.
Figure 2. (A) M1 Group A streptococci 90-226 adherence to A549 cells. (B) Intracellular invasion. Relative adherence and relative invasion were calculated as described in the experimental procedures. Data are the means ± standard errors of the means from three infected monolayer wells. The symbol * represents p < 0.05 between control (CT) and treatment group.
The H35A mutated α-toxin inhibits the toxicity of staphylococcal α-toxin, but had no obvious influence on the cytotoxicity of streptococcal exported toxins
It has been revealed that H35L mutagenesis in α-toxin causes virulence attenuation in vivo.Citation23 We also demonstrated that the H35A mutated α-toxin not only lacks cytotoxicity, but also can neutralize the cytotoxicity of virulent α-toxin.Citation21,Citation24 In order to explore the usage of H35A mutated α-toxin as a potential inhibitor of group A streptococci infection, we examined the toxoid’s role on the inhibition of the cytotoxicity of S. pyogenes. The epithelial cells were either left untreated or pre-treated with H35A α-toxin 30 min prior to exposure of α-toxin or streptococcal supernatant. Consistent with previous studies,Citation24 the exposure of 1 μg/ml of α-toxin led to more than 60% epithelial cell necrosis, however, the pretreatment with H35A mutated α-toxin significantly reduced the toxicity of α-toxin (). Moreover, we found the exposure of A549 epithelial cells to streptococcal supernatant for 16 h caused approximately 30% cell death, whereas the addition of 0.1 μg/ml of H35A α-toxin did not have a significant impact on the cytotoxicity of streptococcal exported toxins (). Furthermore, we did not observe any remarkable impact on the cytotoxicity of the streptococcal supernatant by increasing the doses of H35A α-toxin to 1 μg/ml (data not shown). These data indicate that the H35A mutated α-toxin could not prevent the toxicity of streptococcal toxins to A549 epithelial cells.
Figure 3. Effect of mutated H35A toxin on the capacity of α-toxin- or streptococcal toxins-induced cell death. H35A α-toxin inhibits α-toxin-induced cell death. Monolayers of A549 cells (2 × 105 cells/well) were pretreated for 30 min with 0.1 μg/ml of α-toxin-H35A or control (without any treatment) before exposure to staphylococcal α-toxin or streptococcal toxins. Cell viability was determined by measuring ATP in the cells 16 h after final treatment and is expressed as an average of percentage of cell survival of three experiments ± standard deviation. The symbol * represents p < 0.05 between control (without any treatment) and treatment group of α-toxin, and between α-toxin-treatment group and pretreatment group with H35A toxoid.
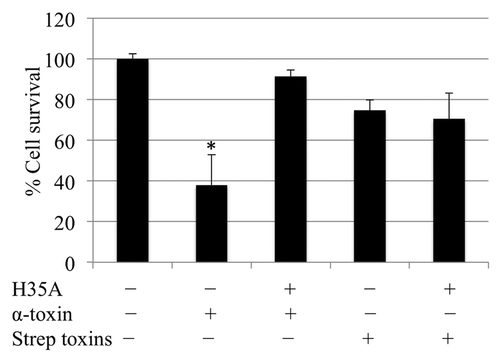
We have demonstrated that H35A mutated α-toxin inhibits the cytotoxicity of wild-type α-toxin by interrupting pore formation.Citation24 Taken together, the above data indicate the different mechanisms of cytotoxicity between staphylococcal α-toxin and streptococcal exported toxins. Although our previous studies have demonstrated that the α5β1-integrin signaling pathway is involved in α-toxin-induced cell death,Citation22 we cannot rule out the possibility that H35A may interfere α-toxin interaction with its receptor ADAM10. It has been revealed that the α-toxin cellular receptor ADAM10 in the epithelia of lung and skin is involved in tissue damage caused by S. aureus infection.Citation25,Citation26 To investigate the impact of ADAM10 signaling pathway on the inhibitory function of H35A mutated toxin is beyond the scope of this study.
In conclusion, S. aureus and S. pyogenes are important human pathogens that can cause a variety of infections. In this report, our results clearly indicate that H35A toxoid inhibits the adhesion and internalization of A549 epithelial cells by a human clinical S. aureus (MRSA) isolate WCUH29 and M1 group A streptococci 90–226. In addition, we found that H35A toxoid inhibits the cytotoxicity of staphylococcal α-toxin, but doesn’t affect the toxicity of streptococcal exported proteins. These findings suggest that the H35A mutated α-toxin may provide new strategies to prevent the staphylococcal and streptococcal infections.
Materials and Methods
Bacterial strains, plasmids and growth media
A clinical human methicillin-resistant S. aureus (MRSA) isolate, WCUH29Citation17 and a M1 serotype S. pyogenes 90-226Citation6 were used for this study. The S. aureus cells were cultured in Trypticase soy broth (TSB) at 37°C with shaking or on solid media containing 1% agar (TSA). Group A streptococci were grown in Todd-Hewitt broth supplemented with 1% yeast extract (THY) medium or on 5% sheep blood agar plates. Both the H35A mutated α-toxin and the wild-type α-toxin were overexpressed and purified from the supernatant of S. aureus cultures as described.Citation21,Citation24
Cell culture and epithelial cell adhesion and invasion assay
A549 human lung epithelial cells (ATCC CCL 185) were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS; Invitrogen). Cultures of A549 cells were maintained in medium containing penicillin (5 µg/ml) and streptomycin (100 µg/ml) (Sigma).
Assays of bacterial invasion and adherence were performed as previously described.Citation6,Citation27 Assays were performed in RPMI 1640 medium with or without supplement such as 10% FBS (RPMI-FBS) or Fn (10 µg/ml). In some experiments, RPMI 1640 medium was supplemented with different doses of α-toxin, antiserum, or different inhibitors. Briefly, one day prior to infection, approximately 2 × 105 cells were seeded in each well of 24-well plates and incubated overnight at 37°C in a 5% CO2 incubator. Monolayers of A549 cells (2 × 105 cells/well) were infected with approximately 5 × 105 CFU of bacteria and incubated for 2 h at 37°C in 5% CO2.
To measure bacterial adherence, culture media were removed from monolayers at the end of the adhesion period and discarded. The monolayer cells were then washed three times with PBS (pH 7.4) to remove non-adherent bacteria. Epithelial cells were dispersed by the addition of 150 µl of 0.25% trypsin-1 mM EDTA (Invitrogen) and then lysed by addition of 400 µl of 0.025% Triton X-100. The numbers of bacterial CFU released from the lysed epithelial cells were determined by plating of diluted lysates on TSA-agar plates. For adherence assay, the numbers of adherent CFU recovered from these wells represents the number of adherent and internalized bacteria. The actual average CFU/well added, percent of adhesion and normalized adhesion were calculated and statistically analyzed by Student’s t-test, using Microsoft Excel 2003 software. p values of < 0.05 were considered significant.
For invasion assay, culture media were collected from wells for total count and discarded from invasion wells after 2 h incubation. The monolayer cells were washed three times with PBS (pH 7.4) followed by the addition of 1 ml of RPMI-10% FCS containing 100 µg/ml gentamicin and 5 µg/ml lysostaphin to invasion wells and 1 ml of RPMI-10% FBS to total wells and incubated for 1 h. After 1 h, the supernatant in the wells designated total count were collected and the rest removed and discarded. The collected supernatant was stored on ice until ready for plating. All wells were washed three times with 1 ml PBS. Wells designated invasion had 1 ml RPMI, 10% FBS, 5 µg/ml lysostaphin and 100 µg/ml gentamicin added to them to kill non-internalized staphylococci and streptococci, respectively; and wells designated total count had 1 ml of RPMI and 10% FBS added. The plate was placed back in incubator for 1 h. The plate was removed and the supernatant from the total count wells was collected and the rest of the media was discarded. All wells were washed three times with 1 ml warm PBS and then 150 µl of 0.25% trypsin and 1 mM EDTA was added. After 5 min the cells in each well was carefully collected and then 400 µl of 0.025% Triton X-100 was added to the tubes on ice. The numbers of bacterial CFU released from the lysed epithelial cells were determined by plating of diluted lysates on TSA-agar plates. The percent invasion and normalized adhesion were calculated and statistically analysis by Student’s t-test, using Microsoft Excel 2003 software. p values of < 0.05 were considered significant.
Cytotoxicity assays
Briefly, epithelial cells were grown in 96-well plates to 70% confluence. To induce lysis, monolayer cells were exposed to α-toxin and streptococcal toxins with or without α-toxin-H35A and incubated for 16 h at 37°C with 5% CO2. At the end of the experiment, cell viability was determined using the CellTiter-Glo Luminescent Cell Viability Assay (Promega) per the manufacturer’s instructions. Each experiment was repeated three times and the percentage of cell death was normalized to the control (no death) were calculated and statistically analyzed by Student’s t-test, using Microsoft Excel 2003 software. p values of < 0.05 were considered significant.
Acknowledgments
This work was partially supported by grant AI065740 and AI057451 from the National Institute of Allergy and Infectious Disease, and partially by the MAES competitive grants. We thank Mr. Jeffrey Hall for technical support and critical reading of the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Steinberg JP, Clark CC, Hackman BO. Nosocomial and community-acquired Staphylococcus aureus bacteremias from 1980 to 1993: impact of intravascular devices and methicillin resistance. Clin Infect Dis 1996; 23:255 - 9; http://dx.doi.org/10.1093/clinids/23.2.255; PMID: 8842259
- von Eiff C, Becker K, Machka K, Stammer H, Peters G, Study Group. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med 2001; 344:11 - 6; http://dx.doi.org/10.1056/NEJM200101043440102; PMID: 11136954
- Lowy FD. Staphylococcus aureus infections. N Engl J Med 1998; 339:520 - 32; http://dx.doi.org/10.1056/NEJM199808203390806; PMID: 9709046
- Stevens DL. Invasive group A streptococcus infections. Clin Infect Dis 1992; 14:2 - 11; http://dx.doi.org/10.1093/clinids/14.1.2; PMID: 1571429
- Bayles KW, Wesson CA, Liou LE, Fox LK, Bohach GA, Trumble WR. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect Immun 1998; 66:336 - 42; PMID: 9423876
- Cue D, Dombek PE, Lam H, Cleary PP. Streptococcus pyogenes serotype M1 encodes multiple pathways for entry into human epithelial cells. Infect Immun 1998; 66:4593 - 601; PMID: 9746555
- Flock JI, Fröman G, Jönsson K, Guss B, Signäs C, Nilsson B, et al. Cloning and expression of the gene for a fibronectin-binding protein from Staphylococcus aureus. EMBO J 1987; 6:2351 - 7; PMID: 2822388
- Rambukkana A, Salzer JL, Yurchenco PD, Tuomanen EI. Neural targeting of Mycobacterium leprae mediated by the G domain of the laminin-α2 chain. Cell 1997; 88:811 - 21; http://dx.doi.org/10.1016/S0092-8674(00)81927-3; PMID: 9118224
- Foster TJ, Höök M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol 1998; 6:484 - 8; http://dx.doi.org/10.1016/S0966-842X(98)01400-0; PMID: 10036727
- Schwarz-Linek U, Werner JM, Pickford AR, Gurusiddappa S, Kim JH, Pilka ES, et al. Pathogenic bacteria attach to human fibronectin through a tandem beta-zipper. Nature 2003; 423:177 - 81; http://dx.doi.org/10.1038/nature01589; PMID: 12736686
- Agerer F, Michel A, Ohlsen K, Hauck CR. Integrin-mediated invasion of Staphylococcus aureus into human cells requires Src family protein-tyrosine kinases. J Biol Chem 2003; 278:42524 - 31; http://dx.doi.org/10.1074/jbc.M302096200; PMID: 12893831
- Wang B, Yurecko RS, Dedhar S, Cleary PP. Integrin-linked kinase is an essential link between integrins and uptake of bacterial pathogens by epithelial cells. Cell Microbiol 2006; 8:257 - 66; http://dx.doi.org/10.1111/j.1462-5822.2005.00618.x; PMID: 16441436
- Menzies BE, Kourteva I. Staphylococcus aureus α-toxin induces apoptosis in endothelial cells. FEMS Immunol Med Microbiol 2000; 29:39 - 45; PMID: 10967259
- Haslinger B, Strangfeld K, Peters G, Schulze-Osthoff K, Sinha B. Staphylococcus aureus α-toxin induces apoptosis in peripheral blood mononuclear cells: role of endogenous tumour necrosis factor-α and the mitochondrial death pathway. Cell Microbiol 2003; 5:729 - 41; http://dx.doi.org/10.1046/j.1462-5822.2003.00317.x; PMID: 12969378
- Kielian T, Cheung A, Hickey WF. Diminished virulence of an alpha-toxin mutant of Staphylococcus aureus in experimental brain abscesses. Infect Immun 2001; 69:6902 - 11; http://dx.doi.org/10.1128/IAI.69.11.6902-6911.2001; PMID: 11598065
- Kernodle DS, Voladri RK, Menzies BE, Hager CC, Edwards KM. Expression of an antisense hla fragment in Staphylococcus aureus reduces alpha-toxin production in vitro and attenuates lethal activity in a murine model. Infect Immun 1997; 65:179 - 84; PMID: 8975909
- Ji Y, Marra A, Rosenberg M, Woodnutt G. Regulated antisense RNA eliminates alpha-toxin virulence in Staphylococcus aureus infection. J Bacteriol 1999; 181:6585 - 90; PMID: 10542157
- Bubeck Wardenburg J, Patel RJ, Schneewind O. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun 2007; 75:1040 - 4; http://dx.doi.org/10.1128/IAI.01313-06; PMID: 17101657
- Bayer AS, Ramos MD, Menzies BE, Yeaman MR, Shen AJ, Cheung AL. Hyperproduction of alpha-toxin by Staphylococcus aureus results in paradoxically reduced virulence in experimental endocarditis: a host defense role for platelet microbicidal proteins. Infect Immun 1997; 65:4652 - 60; PMID: 9353046
- Sadowska B, Bonar A, von Eiff C, Proctor RA, Chmiela M, Rudnicka W, et al. Characteristics of Staphylococcus aureus, isolated from airways of cystic fibrosis patients, and their small colony variants. FEMS Immunol Med Microbiol 2002; 32:191 - 7; http://dx.doi.org/10.1111/j.1574-695X.2002.tb00553.x; PMID: 11934563
- Liang X, Ji Y. Alpha-toxin interferes with integrin-mediated adhesion and internalization of Staphylococcus aureus by epithelial cells. Cell Microbiol 2006; 8:1656 - 68; http://dx.doi.org/10.1111/j.1462-5822.2006.00740.x; PMID: 16984420
- Liang X, Ji Y. Involvement of alpha5beta1-integrin and TNF-alpha in Staphylococcus aureus alpha-toxin-induced death of epithelial cells. Cell Microbiol 2007; 9:1809 - 21; http://dx.doi.org/10.1111/j.1462-5822.2007.00917.x; PMID: 17359518
- Menzies BE, Kernodle DS. Site-directed mutagenesis of the alpha-toxin gene of Staphylococcus aureus: role of histidines in toxin activity in vitro and in a murine model. Infect Immun 1994; 62:1843 - 7; PMID: 8168947
- Liang X, Yan M, Ji Y. The H35A mutated alpha-toxin interferes with cytotoxicity of staphylococcal alpha-toxin. Infect Immun 2009; 77:977 - 83; http://dx.doi.org/10.1128/IAI.00920-08; PMID: 19103771
- Inoshima I, Inoshima N, Wilke GA, Powers ME, Frank KM, Wang Y, et al. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat Med 2011; 17:1310 - 4; http://dx.doi.org/10.1038/nm.2451; PMID: 21926978
- Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, et al. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis 2010; 202:1050 - 8; http://dx.doi.org/10.1086/656043; PMID: 20726702
- Liang X, Yu C, Sun J, Liu H, Landwehr C, Holmes D, et al. Inactivation of a two-component signal transduction system, SaeRS, eliminates adherence and attenuates virulence of Staphylococcus aureus. Infect Immun 2006; 74:4655 - 65; http://dx.doi.org/10.1128/IAI.00322-06; PMID: 16861653