Figures & data
Table 1. Socio-demographics and disease characteristics of the SABINA III Latin American population by investigator-classified asthma severity and practice type.
Figure 1. Patient enrollment across countries in the SABINA III Latin American cohort (N = 1096). SABINA: SABA use IN Asthma.
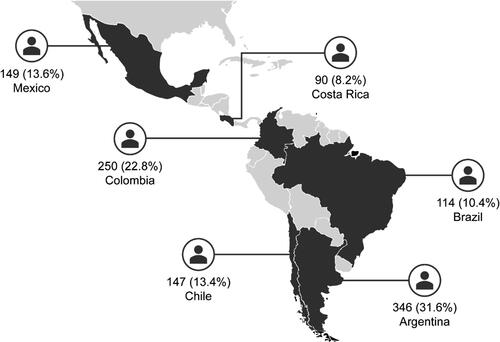
Table 2. Asthma characteristics of the SABINA III Latin American population stratified by investigator-classified asthma severity and practice type.
Figure 2. Proportion of patients receiving SABA prescriptions in the 12 months before the study visit according to investigator-classified asthma severity and practice type in the SABINA III Latin American cohort (N = 1096): (A) all patients, (B) patients with mild asthma, and (C) patients with moderate-to-severe asthma. *The category of patients classified as having 0 SABA canister prescriptions included patients using non–SABA relievers, non-inhaler forms of SABA, and/or SABA purchased OTC. OTC: over the counter; SABA: short-acting β2-agonist; SABINA: SABA use IN Asthma.
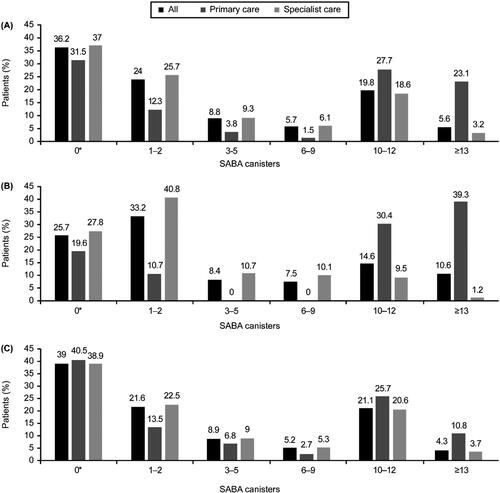
Table 3. Patients in the SABINA III Latin American cohort who (A) received prescriptions for SABA monotherapy, (B) received prescriptions for SABA in addition to maintenance therapy, and (C) purchased SABA without a prescription in the 12 months before the study visit.
Figure 3. SABA OTC purchases and prescriptions in patients with asthma in 6 Latin American countries (n = 188). OTC: over-the-counter; SABA, short-acting β2-agonist.
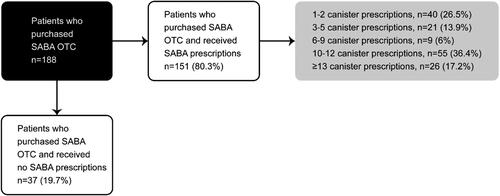
Table 4. Other categories of asthma treatment prescribed in the 12 months before the study visit to patients in the SABINA III Latin American cohort.
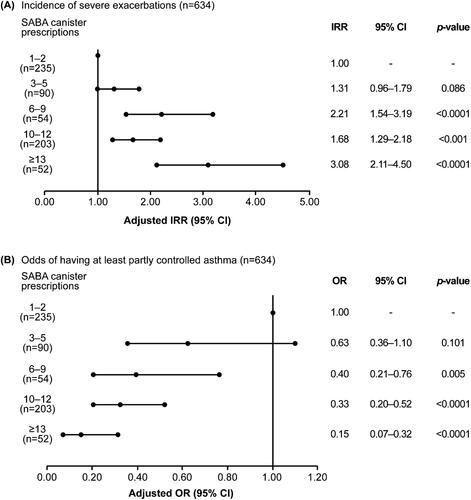
Figure 4. Association of SABA prescriptions with (A) incidence of severe exacerbations in the 12 months before the study visit and (B) level of asthma control assessed during the study visit in the SABINA III Latin American cohort. Based on the co-variable significance in the models, IRRs are corrected by country, age, sex, BMI, smoking history, GINA step, and education level; ORs are corrected by country, age, sex, BMI, asthma duration, smoking history, comorbidity, GINA step, and education level. BMI: body mass index; CI: confidence interval; GINA: Global Initiative for Asthma; IRR: incidence rate ratio; OR: odds ratio; SABA: short-acting β2-agonist; SABINA: SABA use IN Asthma.
Supplementary_material_CLEAN.docx
Download MS Word (396.5 KB)Data availability statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
