Figures & data
Figure 1. The culture supernatant of B. adolescentis with quercetin suppressed the production of the inflammatory mediators from LPS-stimulated macrophages. B. adolescentis was incubated with DMSO (final concentration 0.05%, 0 μM quercetin) or quercetin (25 μM) in serum-free DMEM at 37 °C for 3 h under static and anaerobic conditions. The culture supernatants were prepared by the centrifugation twice, and then added to RAW264 cells. Following 24 h of LPS treatment, (A) NO (left, production level; right, inhibition ratio), (B) PGE2, (C) TNF-α, and (D) IL-1β were measured with the Griess assay or ELISA kits. Additionally, protein levels of iNOS and COX-2 were analyzed using western blotting (E). Intensities of these bands were quantified and corrected with the band intensity of pan-actin as an internal standard. Data are shown as means ± SD (n = 3) and were analyzed for statistically significant differences by multiple comparison testing (Tukey–Kramer test and Student’s t-test). Indicated images are representative data. Different letters indicate significant differences (p < 0.05). BA, B. adolescentis; IL, interleukin; LPS, lipopolysaccharide; PG, prostaglandin; QUE, quercetin; TNF, tumor necrosis factor.
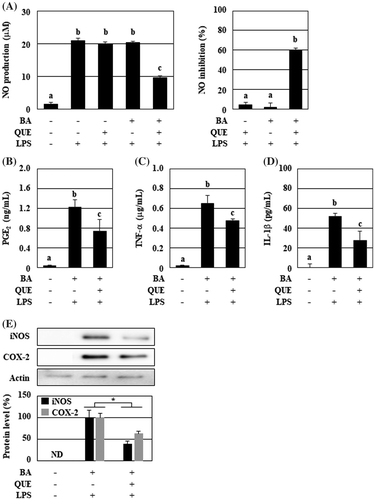
Figure 2. Quercetin, but not decomposed products, persistently enhanced the anti-inflammatory activity of B. adolescentis. The samples used for the experiments were as follows: (left column) B. adolescentis and quercetin were simultaneously added to the medium, followed by incubation for 3 h; (center column) B. adolescentis was added to the quercetin-preincubated medium, followed by incubation for 3 h; (right column) B. adolescentis was incubated with quercetin for 3 h, washed with PBS, and then incubated in fresh medium for a further 3 h. The final concentration of quercetin was 25 μM. DMSO (0.05%) was used as a control. Supernatants of the incubated media were collected and evaluated for suppressive effects on NO production in LPS-stimulated macrophages. Data are shown as means ± SD (n = 3) and were analyzed for statistically significant differences by multiple comparison testing (Tukey–Kramer test). Different letters indicate significant differences (p < 0.05). BA, B. adolescentis; LPS, lipopolysaccharide; QUE, quercetin.
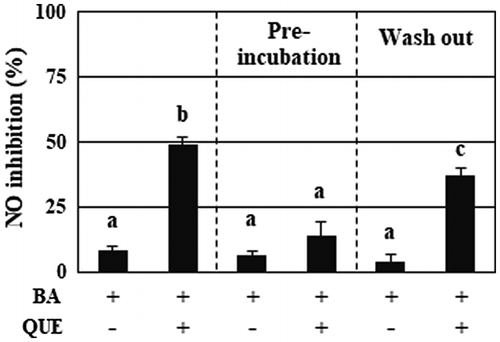
Figure 3. Acetate and lactate had a weak suppression of NO production. RAW264 cells were pretreated with acetate, lactate (final concentration 1.25–20 mM), and a combination for 20 min, and then stimulated by LPS for 24 h. After incubation, the suppressive effects of NO production of the organic acids were evaluated. Data are shown as means ± SD (n = 3). NO, nitric oxide.
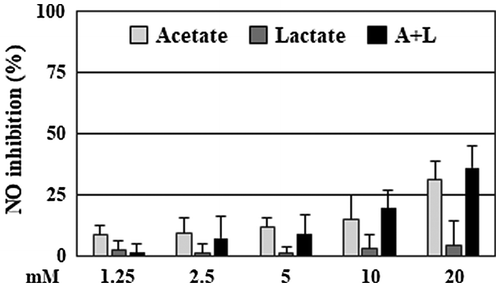
Figure 4. The anti-inflammatory substance of B. adolescentis was a non-phenolic and heat stable compound with low molecular weight. The culture supernatants of B. adolescentis with DMSO or quercetin were treated with (A) Polyclar VT to remove phenolic compounds, (B) heated at 100 °C for 30 min, or (C) fractionated by ultrafiltration as described in the Materials and Methods. Then, the suppressive effects of NO production of the culture supernatant were evaluated. (D) The pH of the culture supernatant was modified by adding hydrogen chloride or sodium hydroxide, and then partitioned by ethyl acetate. The original supernatant (Pre) and the reconstitutes (pH = 2–12: 50-fold concentrates) of the organic layer prepared as described in the Materials and Methods. The reconstitutes were diluted to 1× concentration with serum and antibiotic-free DMEM and then added to RAW264 cells. After 24 h of LPS treatment, the inhibition of the NO production was evaluated. Data are shown as means ± SD (n = 3). BAD, B. adolescentis with DMSO; BAQ, B. adolescentis with quercetin; MW, molecular weight; NO, nitric oxide.
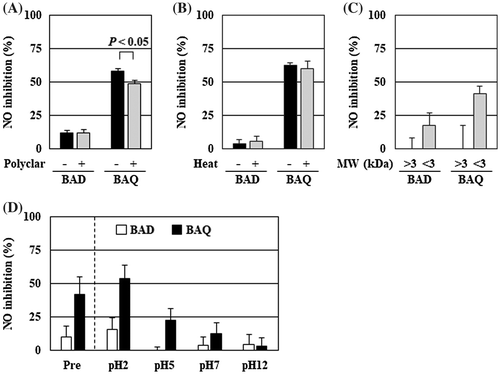
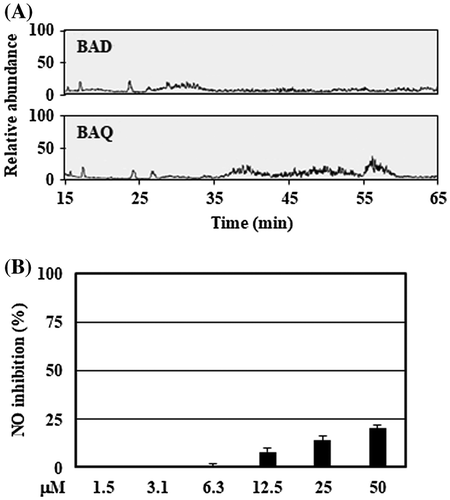
Figure 5. Tentative identification of anti-inflammatory substances showed stearic acid as the biomolecule candidate. (A) The organic layers prepared by the culture supernatant with low pH were analyzed using LC-MS system described in Materials and methods. (B) RAW264 cells were pretreated with stearic acid (final concentration 1.5–50 μM) for 20 min, and then stimulated by LPS for 24 h. After incubation, the suppressive effects of NO production of stearic acid were evaluated. Data are shown as means ± SD (n = 3). BAD, B. adolescentis with DMSO; BAQ, B. adolescentis with quercetin; NO, nitric oxide.