Figures & data
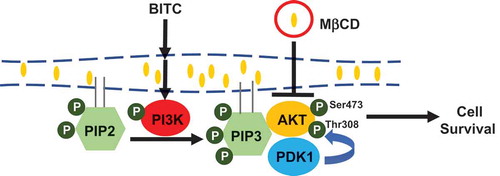
Figure 1. Modulating effects of MβCD on the medium cholesterol and intracellular BITC levels. (a and b) Enhancing effect of MβCD on the medium cholesterol level. The cells were treated with MβCD (0, 1, 2.5, and 5 mM). The cholesterol level in the medium was determined by a TLC analysis. Representative chromatogram (a) and quantitative data (b). PC; phosphatidylcholine. (c) Effect of MβCD on the intracellular BITC level. The cells were treated by MβCD (2.5 mM) for 1 h, then incubated with or without BITC (50 μM) for the indicated periods. Equal quantities of protein samples were subjected the cyclocondensation assay. All values were expressed as means ± SD of three separate experiments (*p < 0.05, **p < 0.01 compared to negative control).
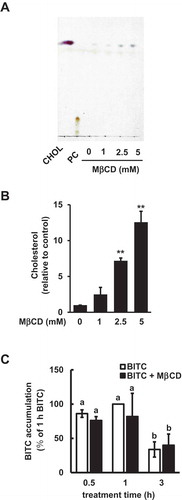
Figure 2. Enhancing effect of MβCD on the BITC-induced antiproliferation. (a) HCT-116 cells were exposed to the indicated concentrations of MβCD for 1 h, then incubated in 1% FBS DMEM medium for 48 h. Cell viability was determined by an MTT assay. All values were expressed as means ± SD of three separate experiments (*p < 0.05, **p < 0.01 compared to negative control). (b) After the pretreatment with 2.5 mM MβCD, the cells were treated with BITC for 48 h. (c) After the pretreatment with 2.5 mM MβCD, the cells were exposed to cholesterol for 1 h, followed by the BITC treatment for 48 h. Cell viability was determined by an MTT assay. All values were expressed as means ± SD of three separate experiments. Different letters above the bars indicate significant differences among the treatments for each condition (p < 0.05).
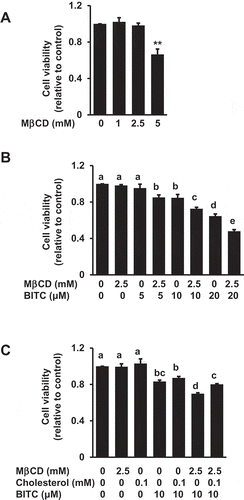
Figure 3. Enhancing effect of MβCD on the BITC-induced apoptotic cell death. HCT-116 cells were pretreated with MβCD (2.5 mM) for 1 h and incubated with or without cholesterol (0.1 mM) for 1 h, followed by the treatment of BITC (10 μM) for 48 h. Apoptosis was detected by an Annexin-V-FLUOS stain kit and analyzed by a Tali™ image-based cytometer. (a) apoptotic cell population (Annexin V positive) and (b) viable cell population (Annexin V negative, propidium iodide negative). All values were expressed as means ± SD of three separate experiments. Different letters above the bars indicate significant differences among the treatments for each condition (p < 0.05).
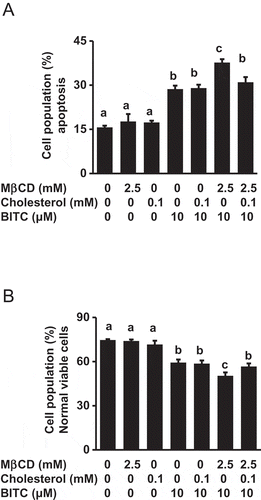
Figure 4. Modulating effect of MβCD on the PI3K/Akt cell survival pathway. (a) HCT-116 cells were pretreated with the indicated concentrations of MβCD for 1 h, then incubated in 1% FBS medium for 30 min. (b) After the pretreatment with MβCD (2.5 mM) for 1 h, the cells were treated with BITC (10 μM) for 30 min. The phosphorylated and total proteins of Akt and PI3K as well as actin were analyzed by Western blotting. All values were expressed as means ± SD of three separate experiments. Different letters above the bars indicate significant differences among the treatments for each condition (p < 0.05).
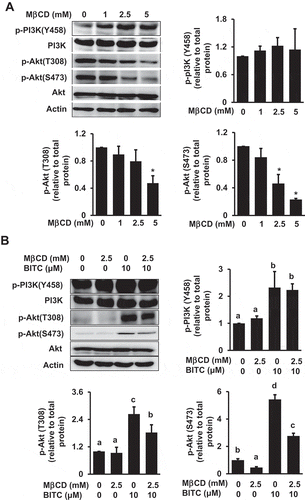
Figure 5. Impairment of the MβCD-induced inhibition of Akt phosphorylation by cholesterol supplementation. HCT-116 cells were pretreated with MβCD (2.5 mM) for 1 h and incubated with or without cholesterol (0.1 mM) for 1 h, followed by the treatment of BITC (10 μM) in the completed medium for 30 min. The phosphorylated and total proteins of Akt as well as actin were analyzed by Western blotting. All values were expressed as means ± SD of three separate experiments. Different letters above the bars indicate significant differences among the treatments for each condition (p < 0.05).
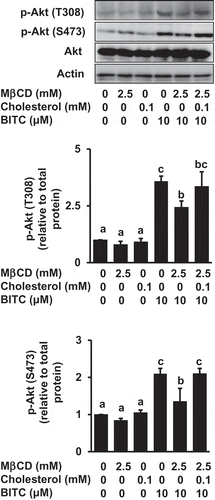
Figure 6. No significant effects of MβCD on the membrane distribution of PDK1 and Akt. After the pretreatment with MβCD (2.5 mM) for 1 h, the cells were treated with BITC (10 μM) for 30 min. Separation of the cytosol and membrane fractions was performed as described in materials and methods section. The total proteins of Akt and PDK1 were analyzed by Western blotting. All values were expressed as means ± SD of three separate experiments. Different letters above the bars indicate significant differences among the treatments for each condition (p < 0.05).
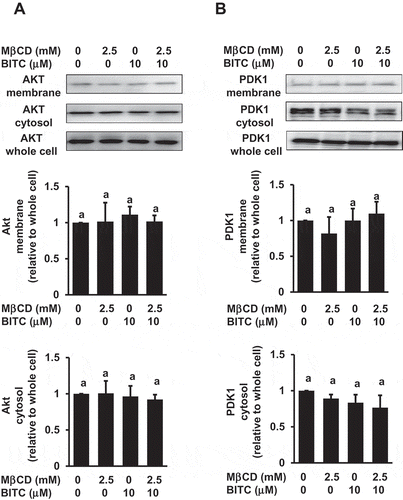
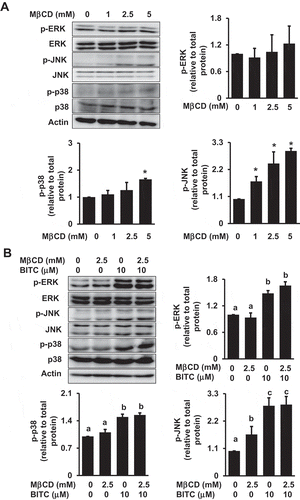
Figure 7. Modulating effects of MβCD on the MAPK pathway. (a) HCT-116 cells were pretreated with the indicated concentrations of MβCD for 1 h, then incubated in 1% FBS medium for 30 min. (b) After the pretreatment with MβCD (2.5 mM) for 1 h, the cells were treated with BITC (10 μM) for 30min. The phosphorylated and total proteins of ERK, JNK, and p38 as well as actin were analyzed by Western blotting. All values were expressed as means ± SD of three separate experiments. Different letters above the bars indicate significant differences among the treatments for each condition (p < 0.05).