Figures & data
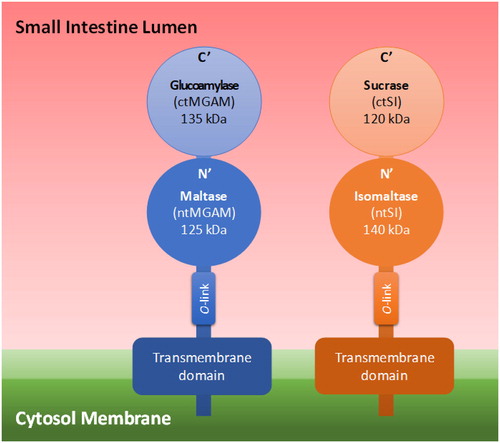
Table 1. Kinetic Parameters of Each Recombinant Mucosal α-Glucosidase on Differently α-Linked Disaccharides with Two Glucoses. Adapted from Lee et al. (Citation2016).
Table 2. α-Glucan ingredients: Chemical structures, properties and applications in food industry.
Figure 4. Topology diagram models of family GH70 Glucansucrases (GS) with a circularly permutated (β/α)8 barrel (a) and the family GH13 α-amylase (β/α)8 barrel (b). Cylinders represent α-helices and arrows represent β-strands. The equivalent α-helices and β-strands in GH70 GSs and GH13 α-amylases are numbered the same. The different domains in GH70 and GH13 enzymes are indicated. Domain C of GH70 GSs is inserted between α-helix 8 and β-strand 1 while that of GH13 family α-amylase locates C-terminally of the (β/α)8 barrel. Domain B of GH13 α-amylases is inserted between β-strand 3 and α-helix 3 while that of GH70 GS is formed by two discontinuous polypeptide segments from both the N- and C-termini. The same is true for domains IV and V of GH70 GS. A variable region (VR) is present in the N-terminus of GH70 GSs. The four conserved sequence motifs (I–IV) which are located in β-strands 3, 4, 5, and 7, respectively, and are shared between family GH70 GS and GH13 enzymes, are indicated within the β-strand. The structure of the catalytic domain in the GH70 GS representative GTF180-ΔN (c, PDB: 3KLK) of L. reuteri 180 and in the GH13 representative α-amylase of Bacillus licheniformis (d, PDB: 1BPL). The (β/α)8 barrel is colored for a better representation. α-Helices and β-strands are numbered, and the conserved sequence motifs (I–IV) are indicated at the corresponding β-strand. The circularly permutated (β/α)8 barrel of GH70 GS is formed by two separate polypeptide segments (N-terminal parts colored in deep blue and C-terminal parts colored in cyan), which is caused by the insertion of domain C.
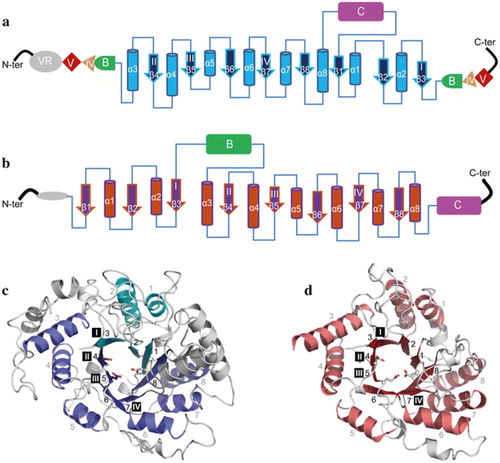
Figure 5. Domain arrangement of sucrose- and starch-converting GH70 enzymes.
Crystal structures of the L. reuteri 121 GtfB 4,6-α-GTase (middle), and the L. reuteri 180 Gtf180 GS (right). Domains A, B, C, IV and V are highlighted in blue, green, magenta, yellow and red, respectively. Ig2-like domains are colored in grey. As apparent from the order of the conserved regions (indicated by grey rectangles), the catalytic barrel of the GH70 glucansucrases and GH70 GtfB-like enzymes is circularly permuted (order = II-III-IV-I).
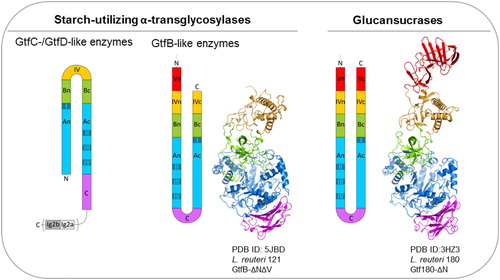
Table 3. Examples of products synthesized by sucrose- and starch-active GH70 enzymes.
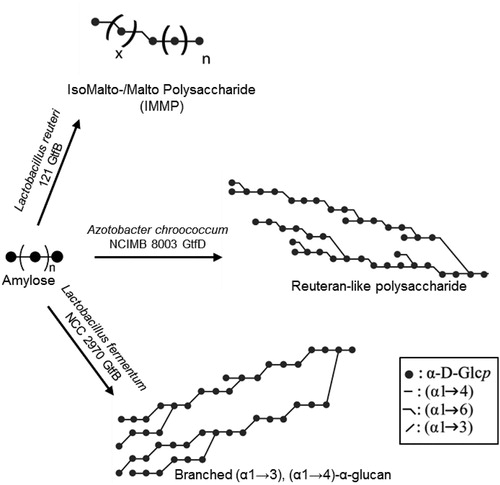
Figure 6. Composite models of representative novel α-glucans synthesized by starch-converting GH70 enzymes.