Figures & data
Figure 1. Structural components of the gut-immune barrier. The gut-immune barrier is a multi-layered structural unit consisting of gut microbiota, the internal mucus layer containing mucins, antimicrobial proteins, and immunoglobulin A (IgA), the epithelial monolayer with enterocytes, goblet cells, Paneth cells, and microfold (M) cells which are connected by tight junctions (TJs); and different immune cells within lamina propria, e.g., macrophages, dendritic cells (DCs), and T-cells. The mucus layer is the small intestine is composed of a single layer while in the large intestine it is composed of two layers. It starts with a loose, outer layer that serves as resident site for the gut microbiota and a firm inner layer in which bacteria are rare. This illustration depicts the barrier in the large intestine.
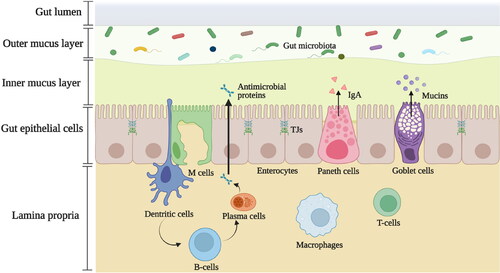
Figure 2. The structures of human milk oligosaccharides (hMOs), pectins, galacto-oligosaccharides (GOS), and inulin-type fructans (ITFs). (A). Main building blocks of hMOs, pectins, GOS and ITFs. (B). HMOs are composed of five building blocks, including glucose (Glc), galactose (Gal), N-acetylglucosamine (GlcNAc), fucose (Fuc), and N-acetylneuramic acid (Neu5Ac). Each hMO possesses a core structure, the Gal-GlcNAc-Gal-Glc unit. The addition of Fuc or Neu5Ac residues can further enrich the core structure of hMOs resulting in neutral hMOs or acid hMOs, respectively. (C). Pectins contain several structural components including homogalacturonan, xylogalacturonan, apiogalacturonan and rhamogalacturona I (RG-I). Homogalacturonan is made up of galacturonic acid (GalA). The GalA residues can be methyl-esterified and are displayed as methyl esters. Xylogalacturonan and apiogalacturonan are the homogalacturonan with attached xylose and apiose residues, respectively. RG-I consists of a backbone in which GalA and rhamnose are alternated, and contain branched chains containing galactose, arabinose, or a combination thereof. (D). GOS are synthesized via the enzymatic trans-galactosylation of lactose by β-galactosidase. GOS are made up of Gal units linked by diverse glycosidic bonds along with a Glc unit at the reducing end. The degree of polymerization (DP) of GOS ranges from 2-8. (E). ITFs mainly consist of fructose (Fn) and a starting Glc unit can be included as well (GFm). The magnitude of n and m determines the DP of ITFs. According to DP values, ITF is classified into inulin (DP = 10-60), oligofructose (DP < 10) derived from the hydrolysis of inulin; and short-chain fructooligosaccharides (scFOS) (DP = 3-6).
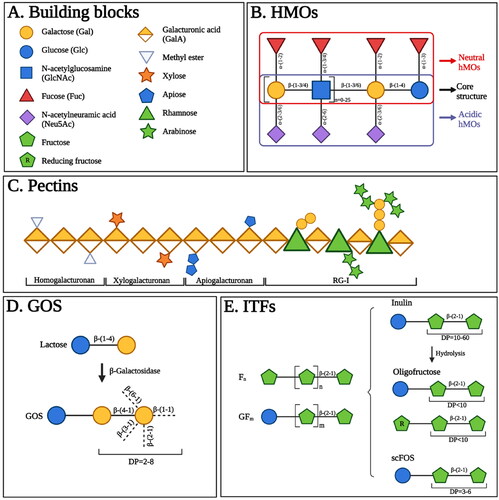
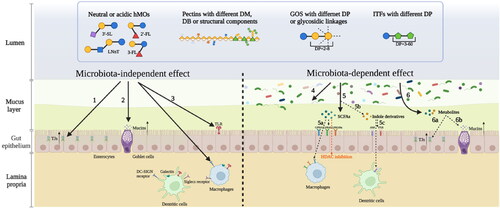
Figure 3. Different structures of individual hMOs and commonly applied NDCs in infant formulas regulate the intestinal immune barrier in a microbiota-independent and microbiota-dependent manner. According to the microbiota-independent effects, they directly 1. support the gut integrity by enhancing tight junctions (TJs); 2. strengthen the mucus layer by stimulating the goblet cells to release mucins; 3. modulate the immune response by interacting with receptors expressed on epithelial cells or immune cells such as Toll-like receptors (TLRs), DC-SIGN receptors, galectin receptors, and Siglecs receptors. These complex carbohydrates can also regulate the intestinal immune barrier in a microbiota-dependent way. 4. They directly modulate the gut microbiota composition. 5. They are fermented by gut microbiota resulting in the production of short-chain fatty acids (SCFAs). 5a. SCFAs exert immunomodulatory effects by interacting with G protein-coupled receptors (GPCRs) or inhibiting histone deacetylases (HDACs). 5b. The intake of hMOs or NDCs may influence the production of indole derivatives. 5c. Indole derivatives also have immunomodulatory functions by interacting with aromatic hydrocarbon receptors (AhR) or pregnancy-X-receptors (PXR). 6. These metabolites such as SCFAs and indoles can improve the gut barrier by upregulating TJs (6a) and enhance the mucus layer by increasing mucins secretion (6b).