Figures & data
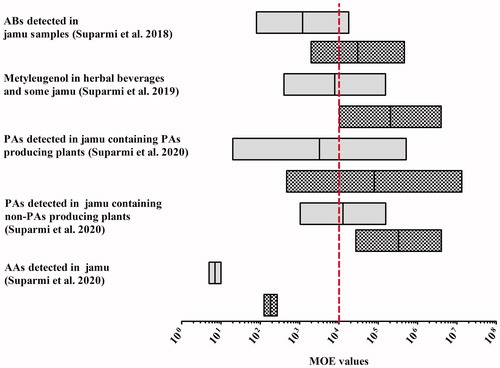
Table 1. Reported adulteration of jamu with APIs and associated hazards and health risks.
(2)
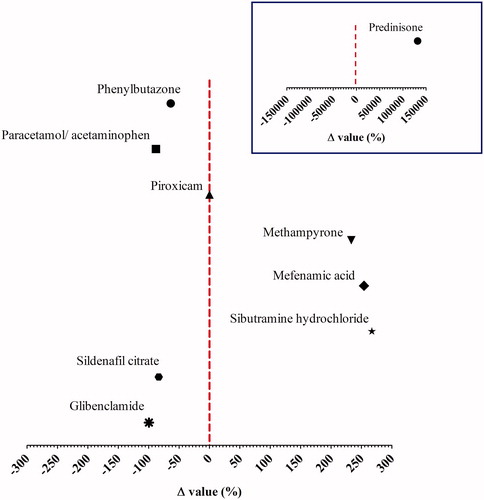
(2) , using the EDIs and the existing reference doses (RD) (Supplemental material 1). The vertical dashed line represents the RD value of 0 as a threshold for risk evaluation. The inserted graph shows the Δ value for prednisone. Note that there is a different X axis.