Figures & data
Table 1. Combination of independent variables in VNP emulsomes experimental runs prepared according to 3221 full factorial design and their corresponding responses.
Table 2. Model fit statistics for the responses of VNP emulsomes prepared according to 3221 full factorial design.
Figure 1. Response 3D surface plot for the influence of PL:SL weight ratio (X1), PL:CH molar ratio (X2), and SL type (X3) on VS (Y1) of VNP emulsomes. (A) SL type (Tripalmitin), (B) SL type (Tristearin), (C) PL:CH (4:1), and (D) PL:SL (2:1).
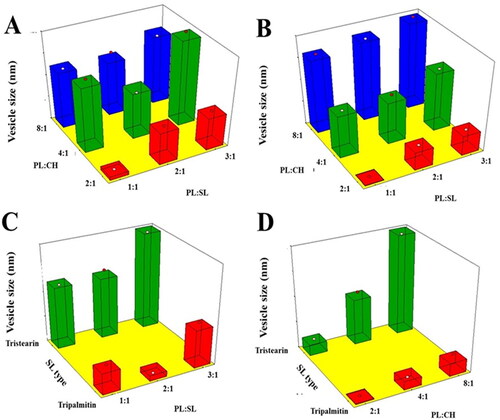
Figure 2. Response 3D surface plot for the influence of PL:SL weight ratio (X1), PL:CH molar ratio (X2), and SL type (X3) on ZP (Y2) of VNP emulsomes. (A) SL type (Tripalmitin), (B) SL type (Tristearin), (C) PL:CH (4:1), and (D) PL:SL (2:1).
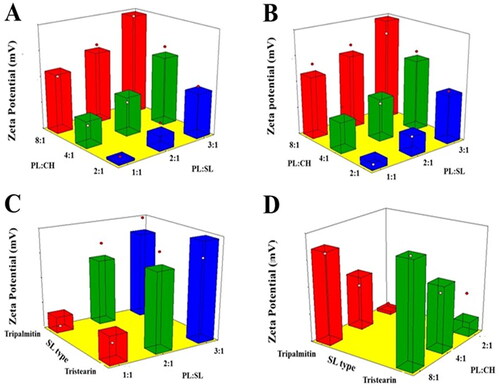
Figure 3. Response 3D surface plot for the influence of PL:SL weight ratio (X1), PL:CH molar ratio (X2), and SL type (X3) on EE% (Y3) of VNP emulsomes. (A) SL type (Tripalmitin), (B) SL type (Tristearin), (C) PL:CH (4:1), and (D) PL:SL (2:1).
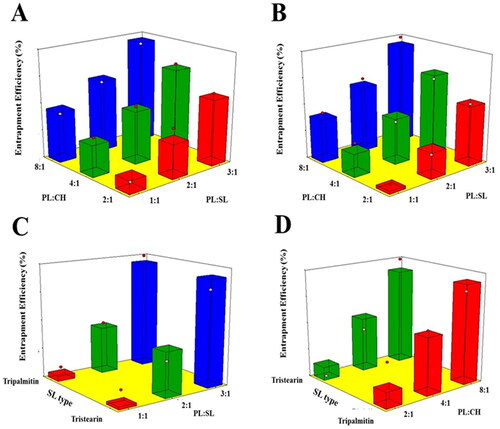
Figure 4. Response 3D surface plot for the influence of PL:SL weight ratio (X1), PL:CH molar ratio (X2), and SL type (X3) on RE24h (Y4) of VNP emulsomes. (A) SL type (Tripalmitin), (B) SL type (Tristearin), (C) PL:CH (4:1), and (D) PL:SL (2:1).
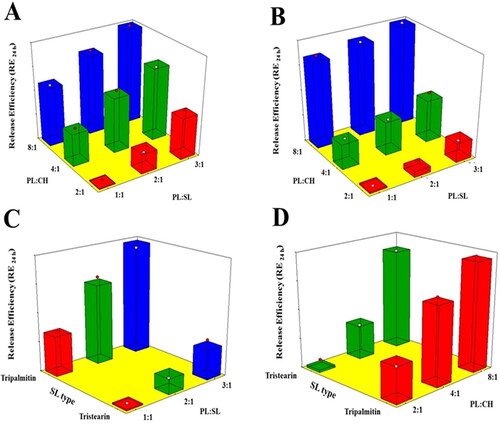
Figure 5. TEM micrographs of (A) optimized VNP emulsomes E12, (B) SA surface-tailored emulsomes E19, and (C) MPEG-DSPE surface-tailored emulsomes E24. Magnification = 22500X.
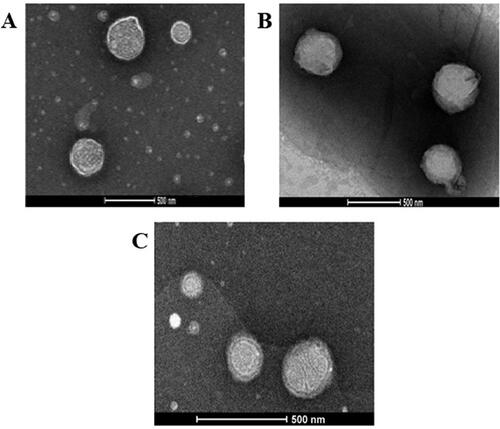
Figure 6. Release profile of (A) SA and (B) MPEG-DSPE surface-tailored emulsomes in PBS (pH 6.8) at 35 ± 0.5 °C compared to the optimized emulsomes (E12). Results are presented as mean (n = 3) ± SD.
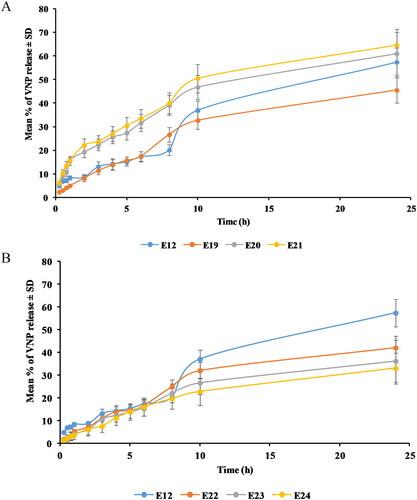
Figure 7. Mean VNP concentration in (A) plasma and (B) brain following the intranasal administration of VNP optimized emulsomes (E12), SA surface-tailored emulsomes (E19) and MPEG-DSPE surface-tailored emulsomes (E24) compared to the orally administered market tablets in rats. Results are presented as mean (n = 6) ± SD.
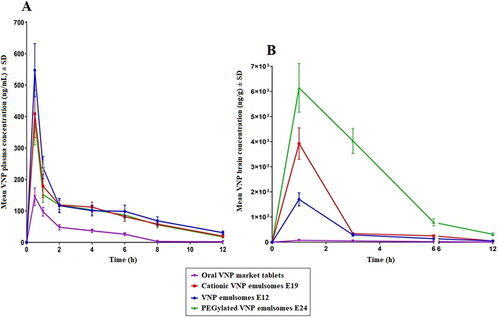
Table 3. Measured plasma and brain pharmacokinetic parameters of VNP following the intranasal administration of E12, E19, and E24 in comparison with the orally administered market tablets.
