Figures & data
Figure 1 Effect of protein concentration on the amount of protein precipitation at pH 4.9 in 0.5 M NaCl.
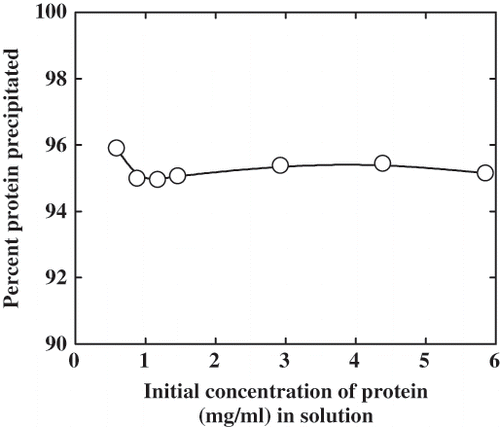
Figure 2 Effect of glycerol on the isoelectric precipitation of α-globulin. The value P/P0 is the ratio of the precipitation in presence of the cosolvent to the precipitation in the control. A protein concentration of 5.6 × 10−6 M was used. (A) From pH 4.0 to 4.9, and (B) from pH 7.0 to 4.9.
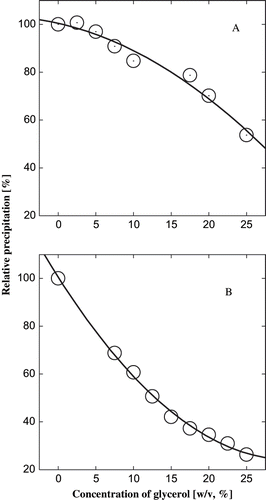
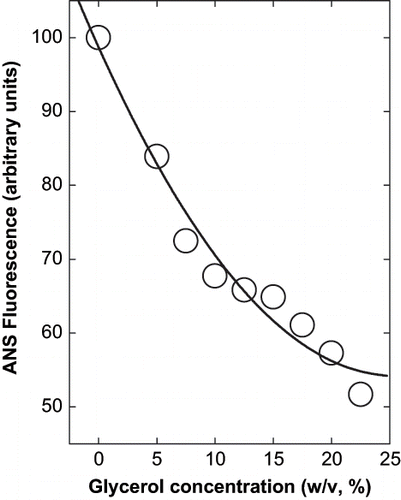
Figure 3 Effect of glycerol on the fluorescence spectra of α-globulin at pH 4.0 (0.02 M sodium acetate buffer). (A) Effect on relative fluorescence intensity; and (B) effect on wavelength of maximum emission. The excitation was at 280 nm and a protein concentration used was 3.8 × 10−7 M.
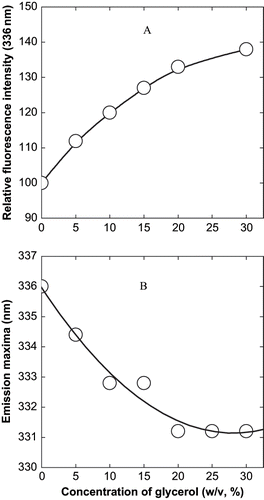
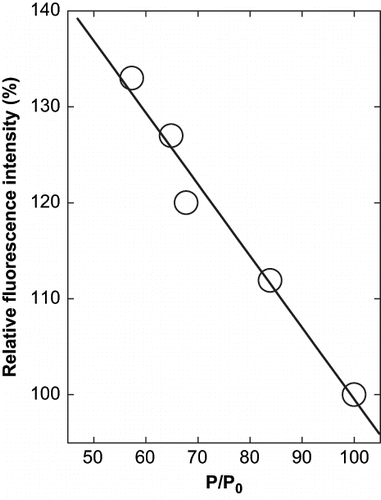
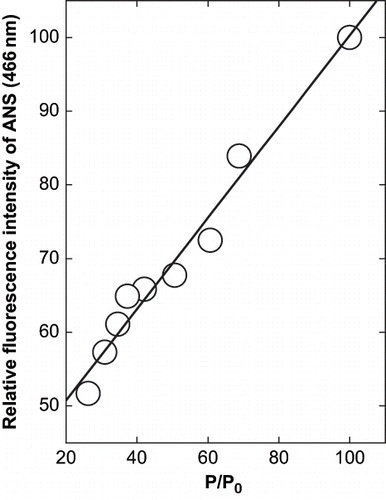
Figure 4 Relation between the precipitation of α-globulin and the change in fluorescence intensity at emission maxima of α-globulin in presence of glycerol at pH 4.0 (0.02 M sodium acetate buffer). The values are plotted from the data of and .