Figures & data
Table 1 Range of values for the response surface methodology
Figure 1 ACE-inhibitory activity in vitro and degree of hydrolysis of soybean protein hydrolysates prepared with different hydrolysis times. The concentration of soybean protein hydrolysates for ACE-inhibitory activity assay was 1 mg/mL on a peptide basis. The bars were for the ACE-inhibitory activity of the hydrolysates and the solid line was for degree of hydrolysis. Different capital letters A to E (or lowercase letters a to g) above the columns (or below the line) indicate that one-way ANOVA of means obtained are significantly different (P < 0.05).
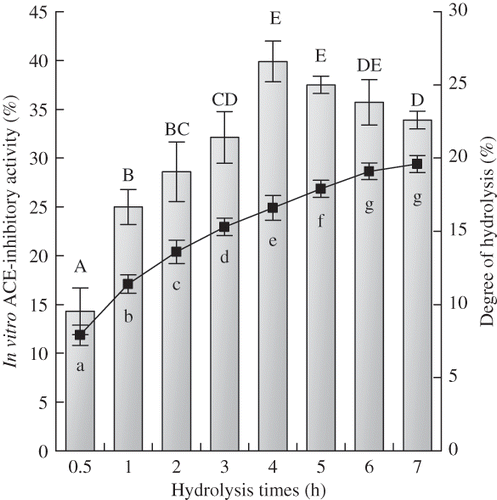
Table 2 ANOVA response for linear, quadratic, and interactive effect of variables used in the model.Footnote a
Figure 2 Response surface graphs for the decreased amount of free amino groups of the modified products of plastein reaction as a function of: (a) E/S ratio and substrate concentration (reaction time at the central of its level), (b) substrate concentration and reaction time (E/S ratio at the central of its level), and (c) E/S ratio and reaction time (substrate concentration at the central of its level). (Color figure available online.)
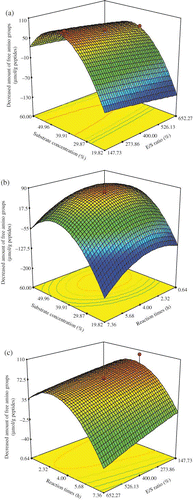
Table 3 The ACE-inhibitory activities of soybean protein hydrolysates (SPH) and eight modified products (MP) of plastein reaction in vitro
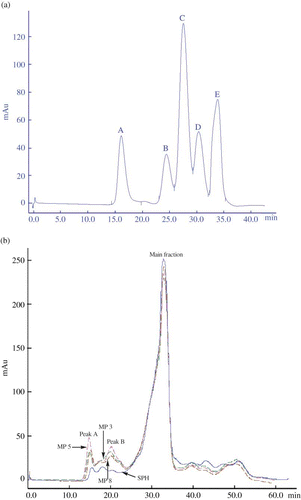
Figure 3 Analysis of the standards (a), soybean protein hydrolysates (SPH) and three modified products (MP3, MP5, and MP8) (b) by size exclusion chromatography in an AKTA Explorer 100 equipped with a Superdex-75 column. The analysis was performed at a flow rate of 0.5 mL/min with 0.1 mol/L Na2HPO4–0.1 mol/L NaOH buffer (pH 12) and monitored at 280 nm. The DH of SPH was 16.6% and the decrease amount of free amino groups of MP3, MP5, and MP8 were 84.70, 75.79, and 44.58 μmol/g peptides, respectively. The standards were bovine serum albumin (66.2 kDa), cytochrome c (12.4 kDa), insulin (5.7 kDa), oxidized L-glutathione (0.6 kDa), and L-tyrosine (0.2 kDa), and appeared as peaks A to E in Fig. 3a, respectively. (Color figure available online.)