Figures & data
Table 1 Purification of polyphenol oxidase from Agaricus bisporus
Figure 1 SDS-PAGE analysis of PPO fractions from each purification step. M: marker; Lane 1: crude extract; Lane 2: pellet by 30–70% (NH4)2SO4; Lane 3: fraction from DEAE-Sepharose Fast Flow; Lane 4: fraction from Phenyl Sepharose CL-4B.
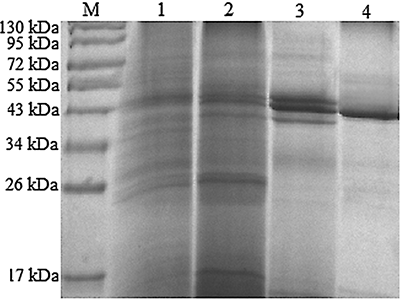
Figure 2 Characterization of PPO activity from Agaricus bisporus. (a) Effect of temperature on the PPO activity of Agaricus bisporus. (b) Heat inactivation of PPO at different temperatures using catechol as substrate. (c) Determination of the optimum pH of the PPO activity from Agaricus bisporus measured using catechol as substrate. (d) Effect of metal ions on the activity of PPO from Agaricus bisporus. (Color figure available online.)
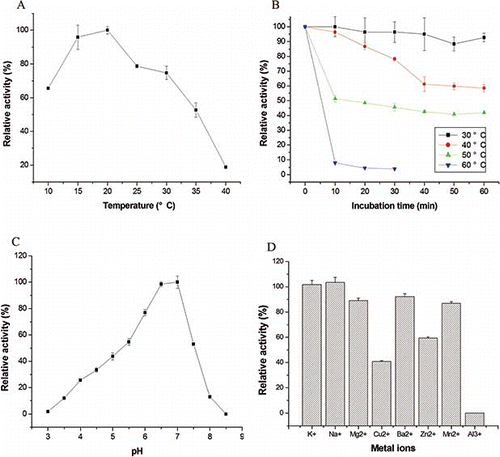
Figure 3 The Lineweaver–Burk plots of PPO activity measured with catechol as substrate. The concentration of catechol was 1, 2, 3, 4, 5, and 6 mM, respectively. (Color figure available online.)
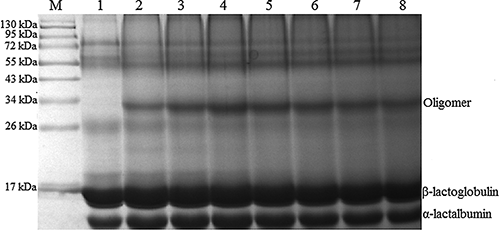
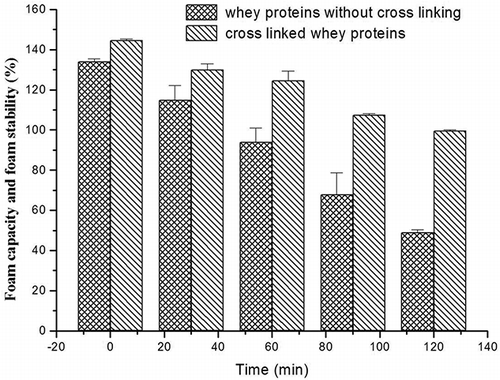
Figure 4 Effect of PPO activity on the cross linking of whey proteins in the presence of caffeic acid. The conditions of cross linking were as follows: caffeic acid 1 mM, pH 4, 40°C for 4 h, PPO activity 200–1400 U/ml. M: marker; Lane 1: control sample; Lane 2: 200 U/ml; Lane 3: 400 U/ml; Lane 4: 600 U/ml; Lane 5: 800 U/ml; Lane 6: 1000 U/ml; Lane 7: 1200 U/ml; Lane 8: 1400 U/ml.