Figures & data
Figure 1 Non-denaturing polyacrylamide gel electrophoresis. β-Glucosidase bands were visualized under UV light after necessary procedures. (a) crude enzyme extract and (b) partially purified L. pyriforme β-glucosidase.

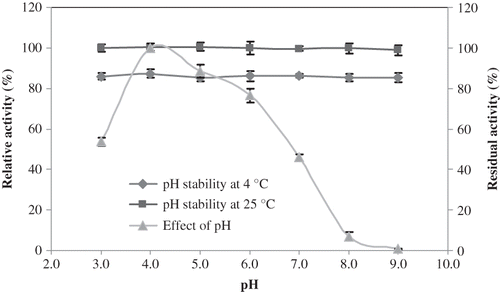
Figure 3 Temperature-activity profile of L. pyriforme β-glucosidase. The background hydrolysis of the substrate was deducted by using a reference sample of identical composition to the incubation mixture except for the enzyme. The activity was expressed as percent relative activity in relation to the temperature optimum, which was considered as 100%.
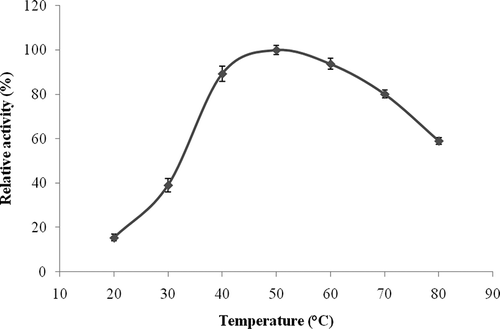
Figure 4 Lineweaver-Burk plot of L. pyriforme β-glucosidase. Enzyme kinetic parameters of the enzyme were obtained by measuring the rate of pNPG hydrolysis at various substrate concentrations at 50°C in 50 mM acetate buffer (pH 4.0).
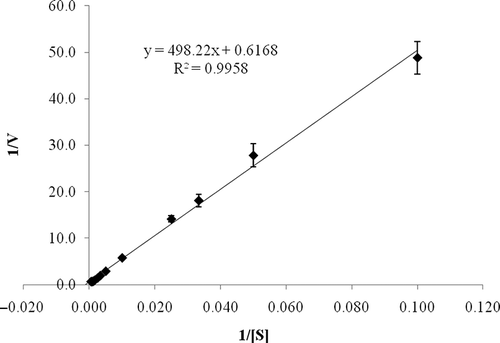
Table 1 Effect of some metal ions on the β-glucosidase activity of L. pyriforme. Enzyme activity determined in the absence of metal ion was defined as 100%
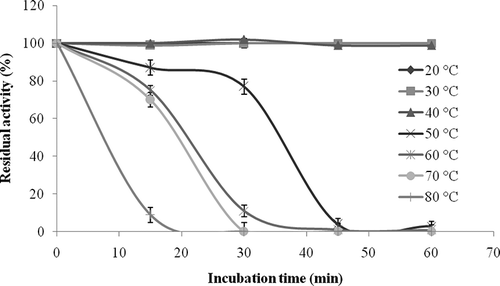
Figure 5 Thermal stability profile of L. pyriforme β-glucosidase. Thermal stability of the enzyme was studied by incubating the enzyme in Eppendorf tubes over the range of 20–80°C. Control with non-incubated enzyme was used to determine the 100% activity value.