Figures & data
FIGURE 1 Schematic representation of sampling plan. For each produce type (e.g., raspberries), three independent samples of each cultivation variety were subjected to organic extraction, and the extracts analyzed by DPV.
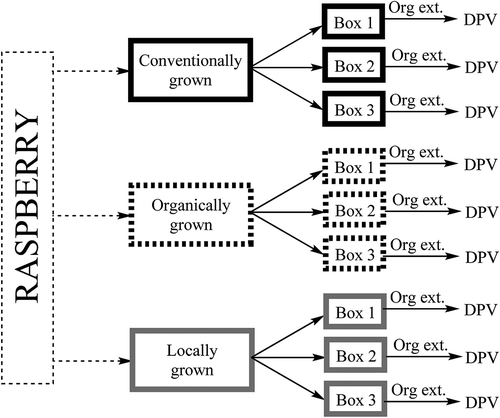
FIGURE 2 Calibration curve expressing voltammogram peak area in microwatts (µW) as a function of gallic acid (GA) concentration; n = 6, (GA) = 4.5 × 10−3 to 1.1 × 10−6 mol/L.
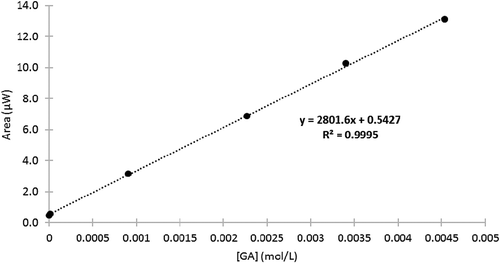
FIGURE 3 Representative differential pulse voltammograms for conventionally (solid line), locally (dotted line), and organically (dashed line) grown produce samples, showing current (µA, y-axis) as a function of applied voltage (mV, x-axis).
TABLE 1 Potential (Ep) and area (A, measured in nanowatts) of principal peak in differential pulse voltammograms of analyzed fruits and vegetables. Values are shown as the mean ± standard deviation (n = 3)
TABLE 2 Antioxidant content of samples in gallic acid equivalents, based on gallic acid calibration curve. Values are shown as the mean ± standard deviation (n = 3)
TABLE 3 Validation parameters of DPV method. Parameters derived from calibration curve of gallic acid in 0.1 mol/L phosphate buffer at pH 2.0 at a glassy carbon electrode. Repeatability of peak potential, height, and area are based on three independent scans of a 2.27 × 10−3 mol/L solution
