Figures & data
FIGURE 1 Effect of various concentrations of ferulic acid on the visible light spectral characteristics in the model system. (1): control samples (anthocyanins without ferulic acid; λmax = 515 nm and Amax = 0.557); (2), (3), and (4): test samples (anthocyanins with ferulic acid) with various concentrations (2, 4, and 6 mg mL–Citation1, respectively) of ferulic acid.
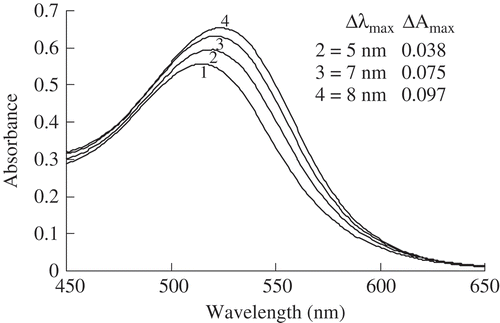
FIGURE 2 Effect of ferulic acid on the visible light spectral characteristics of the model system initially and after 90 days of storage at 25°C while protected from light. (1): control samples (anthocyanins without ferulic acid); (2): samples enhanced with ferulic acid. The concentration of ferulic acid was 6 mg mL–Citation1. (A): before storage; (B): after 90 days of storage.
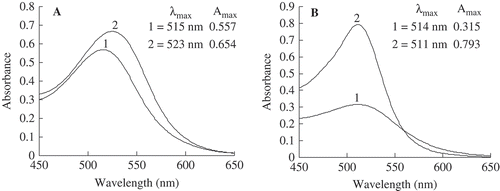
TABLE 1 Effect of ferulic acid on the color changes of anthocyanins from purple corn cob at pH 3.0, initially and after 90 days storage at 25°C protected from light
TABLE 2 Identification of the pigment compounds of test samples by HPLC-MS before and after 90 days storage
FIGURE 3 Changes in HPLC profiles of test samples at the beginning and end of storage. (A): before storage; (B): after 90 days of storage. (1): cyanidin-3-glucoside; (2): pelargonidin-3-glucoside; (3): peonidin-3-glucoside; (4): cyanidin-3-(6-malon-glucoside); (5): pelargonidin-3-(6-malon-glucoside); (6) and (7): peonidin-3-(6-malon-glucoside).
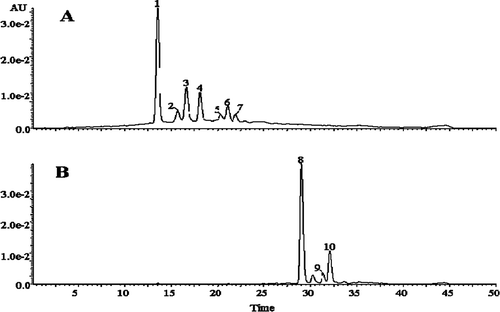
FIGURE 4 Positive ion mass spectra and molecular structure of the novel co-pigmentation compounds formed in the test sample and detected after 90 days of storage. Peak 8: cyanidin-3-glucosede-vinyguaiacol; Peak 9: pelargonidin-3-glucosede-vinylguaiacol; Peak 10: peonidin-3-glucosede-vinylguaiacol; Glu: glucoside.
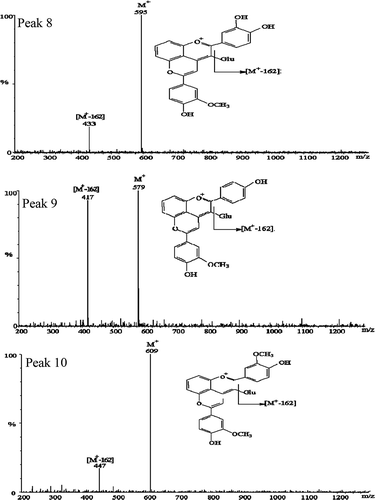
FIGURE 5 The proposed pathway of formation of the new pyranoanthocyanin adduct with anthocyanin-3-glucoside and ferulic acid; Glu: glucoside.
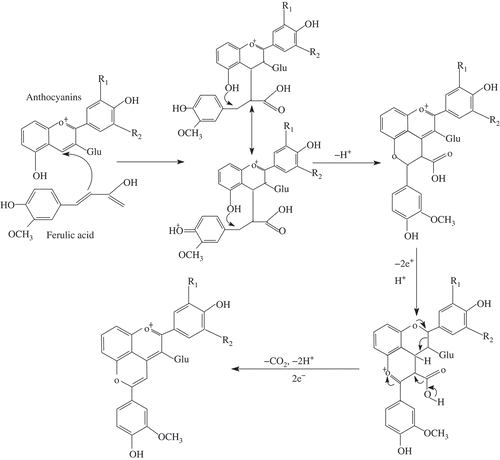
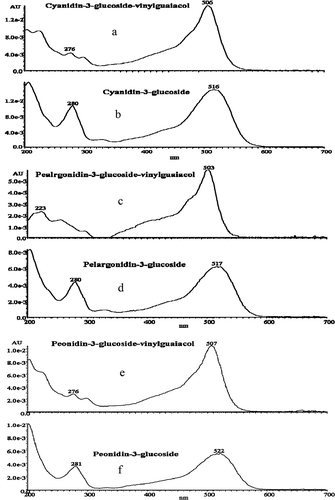
FIGURE 6 Absorption spectrum of (a) cyanidin-3-glucoside-vinylguaiacol; (b) cyanidin-3-glucoside; (c) pelargonidin-3-glucoside-vinylguaiacol; (d) pelargonidin-3-glucoside; (e) peonidin-3-glucoside-vinylguaiacol; and (f) peonidin-3-glucoside.