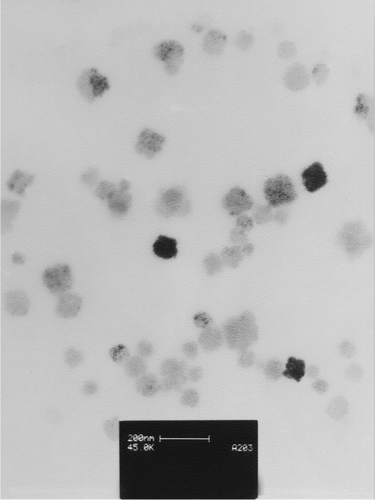
Figures & data
Table 1. Factors affecting formulation and stability of LNC prepared by PIT method.[Citation20]
Figure 1. Schematic production method of lipid nanocapsules by phase-inversion temperature (PIT) technique and chemical structure of LNC and its components.
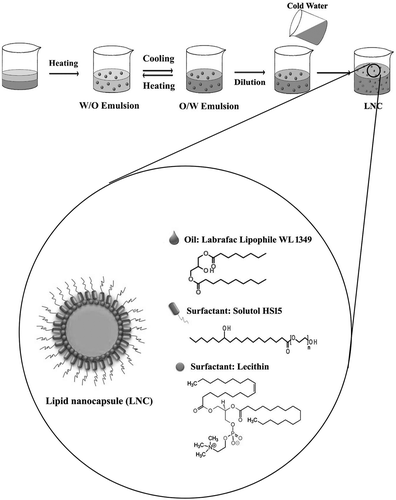
Table 2. Formulation code of vitamin D loaded LNCs.
Table 3. Particle size, polydispersity index (PDI), zeta potential, encapsulation efficiency (EE), and encapsulation load (EL) of produced nanocarriers.
Figure 3. FTIR spectra for vitamin D loaded LNC, blank LNC, Labrafac, Solutol, lecithin, and vitamin D.
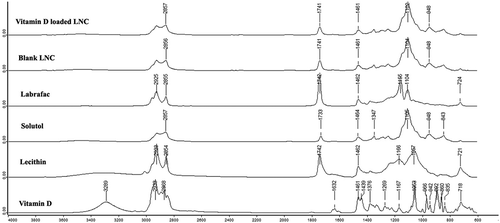
Figure 4. Release profiles of vitamin D loaded LNC at gastric media (pH: 1.2) and intestinal media (pH: 7.4).
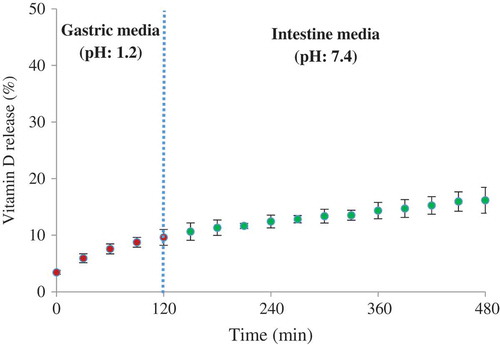
Table 4. Model parameters of vitamin D release from LNC.
Table 5. Sensory results of fortified milk with vitamin D loaded LNC, direct fortification, and blank samples.