Figures & data
Table 1. Substrate specificity of Geobacillus sp. TF16 immobilized phytase.
Figure 1. (a) Effect of pH on Geobacillus sp. TF16 phytase immobilized in chitosan and Ca-alginate. Activity was assayed at 55°C by using sodium phytate as a substrate and 50 mM buffers: glycine–HCl (pH 2.0), Mcilvaine (pH 3.0–7.0), and glycine–NaOH (pH 8.0–9.0). The maximum activity was taken as 100%, and the relative activities were calculated by comparing with it. (b) The optimum temperatures of the enzymes were determined by measuring the activity at temperatures between 25°C and 95°C by using sodium phytate as substrate. The maximum activity was taken as 100% and the relative activities were calculated by comparing with it.
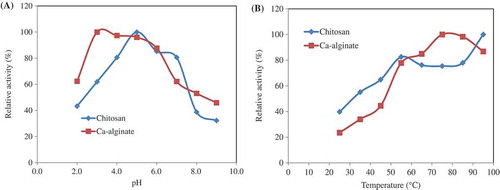
Figure 2. The pH stability of the chitosan-immobilized enzyme was determined at 4°C (a), 35°C (b), and 95°C (c) for different incubation times in the buffer solutions of pH 2.0, pH 5.0, and pH 7.0. At the end of the incubation period, the phytase activity was assayed at optimum conditions. The residual activity (%) was calculated by comparison with unincubated phytase.
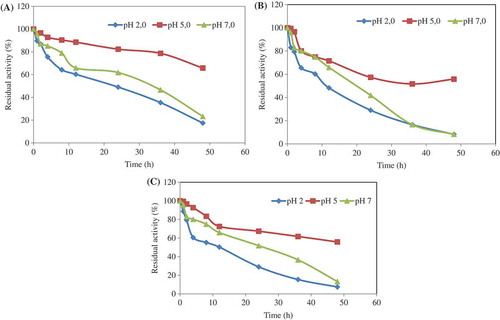
Figure 3. pH stability was separately determined by incubating the enzyme entrapped in alginate/CaCl2 beads at 4°C (a), 35°C (b), and 75°C (c) for different incubation times in the buffer solutions of pH 3.0, pH 5.0, and pH 7.0. At the end of the incubation period, the phytase activity was assayed at optimum pH and temperature values. The residual activity (%) was calculated by comparison with unincubated enzyme.
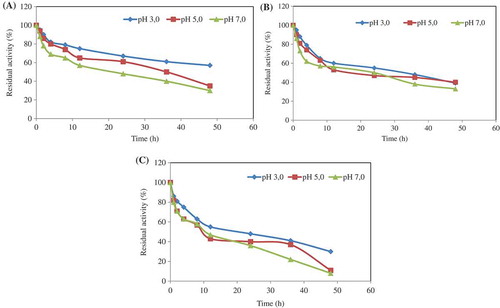
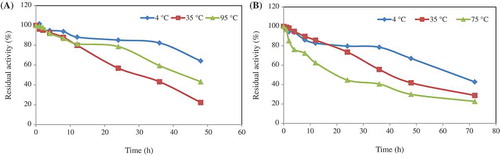
Figure 4. In order to determine the thermal stability of the phytase, the enzyme solutions in 50 mM (pH 5.0) sodium acetate buffer were separately incubated at 4°C, 35°C, and 95°C chitosan (a), and 4°C, 35°C, and 75°C for Ca-alginate (b) at different times. The residual activities (%) were calculated by comparison with standard assay mixture containing unincubated enzyme.