Figures & data
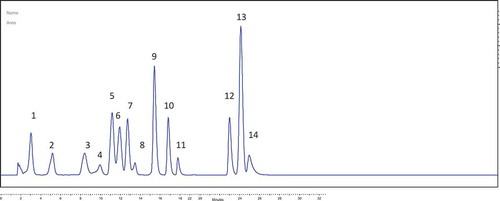
Table 1. RP-HPLC-UV validation parameters of the phenolic compounds in P. spinosa L.
Figure 1. Native PAGE electrophoresis gel revealed by incubating with 100 mM of L-3,4-dihydroxyphenylalanine (L-DOPA) as a substrate of polyphenol oxidase. 1: Crude enzyme after acetone precipitation. Each isoenzyme band was marked.

Table 2. Substrate specificity and activity (U/mg protein) of P. spinosa polyphenol oxidase.
Table 3. Some biochemical parameters of P. spinosa polyphenol oxidase towards various substrates.
Figure 2. The Michaelis–Menten equation of diphenolase activity of P. spinosa for various substrates. (A) 4-methylcatechol (0.05–0.80 mM), (B) catechol (0.10–1.50 mM), (C) hydrocaffeic acid (0.15–5.00 mM), (D) catechin (1.25–15.00 mM), and (E) epicatechin (0.31–10.00 mM) substrates were analysed as 1/specific activity (1/V) versus 1/substrate concentration (1/[S]). The Michaelis–Menten constants (Km) and maximum velocity (Vmax) were obtained from the Lineweaver–Burk plots.
![Figure 2. The Michaelis–Menten equation of diphenolase activity of P. spinosa for various substrates. (A) 4-methylcatechol (0.05–0.80 mM), (B) catechol (0.10–1.50 mM), (C) hydrocaffeic acid (0.15–5.00 mM), (D) catechin (1.25–15.00 mM), and (E) epicatechin (0.31–10.00 mM) substrates were analysed as 1/specific activity (1/V) versus 1/substrate concentration (1/[S]). The Michaelis–Menten constants (Km) and maximum velocity (Vmax) were obtained from the Lineweaver–Burk plots.](/cms/asset/348fd330-60d6-47f1-9017-ddc68d2386e4/ljfp_a_1343349_f0002_b.gif)
Figure 3. pH-activity profile for P. spinosa polyphenol oxidase in the presence of various substrates; (A) 4-methylcatechol, (B) catechol, (C) hydrocaffeic acid, (D) catechin, and (E) epicatechin, and (F) pH stability of P. spinosa polyphenol oxidase at 4°C, at pH 4.0, 5.0, 6.0, and 7.0, incubated in the presence of 4-methylcatechol as a substrate for 24 days.
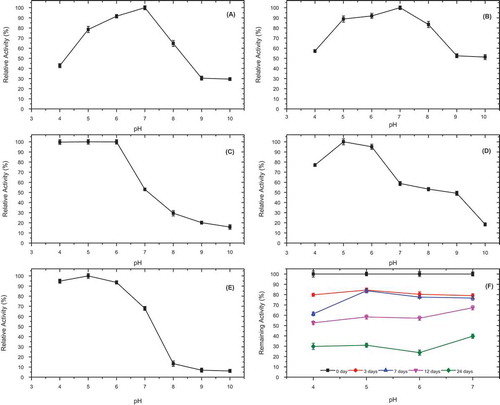
Figure 4. Optimum temperature of P. spinosa polyphenol oxidase in the presence of various substrates; (A) 4-methylcatechol, (B) catechol, (C) hydrocaffeic acid, (D) catechin, and (E) epicatechin, and (F) thermal stability of P. spinosa polyphenol oxidase at 30, 40, and 60°C, at pH 7.0, for 60 min.
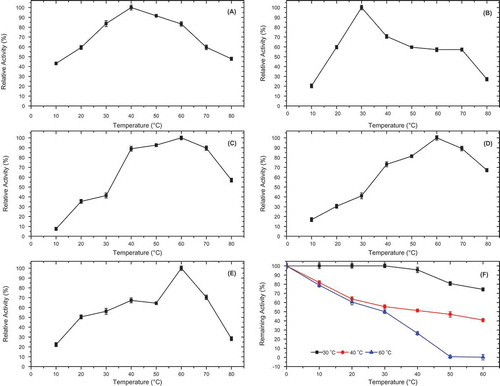
Table 4. The IC50 values of several inhibitors on the diphenolase activity of P. spinosa polyphenol oxidase using 4-MTC as substrate.
Figure 5. Inhibition effect of common polyphenol oxidase inhibitors on the diphenolase activity of P. spinosa. (A) Ascorbic acid (12.50–200 µM), (B) sodium azide (6.25–200 mM), (C) benzoic acid (1.56–50 mM), (D) sodium metabisulphite (3.13–50 µM), (E) thiourea (0.78–25 mM), and (F) L-cysteine (6.25–200 mM).
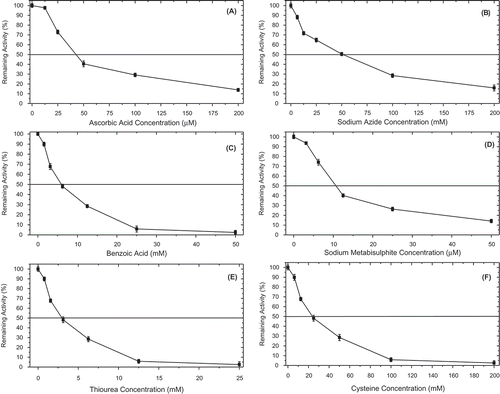
Table 5. Effect of various metal ions on P. spinosa polyphenol oxidase activity in the presence of 4-MTC as substrate.
Table 6. Phenolic acid content of Prunus spinosa L. plum.