Figures & data
Figure 2. Changes of total phenolic and total flavonoid contents of walnut green husks during different stages of development. Note: Results represent the means ± standard deviation (n = 3). Different little letter near the bar indicates significantly difference at p < 0.05 level.
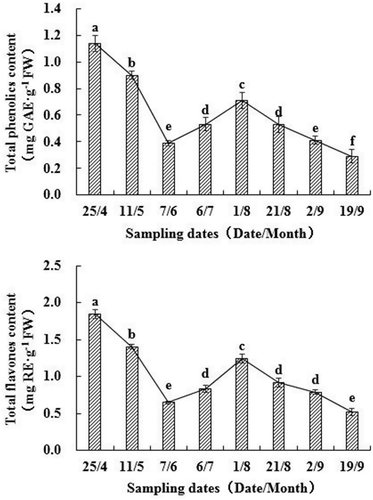
Table 1. Changes of phenolic compounds of walnut green husks during developmental stages (mg/100 g).
Figure 3. HPLC chromatogram of phenolic standards at 280 nm (A) and 251 nm (B). Peaks: 1 gallic acid; 2 catechin; 3 chlorogenic acid; 4 vanillic acid; 5 caffeic acid; 6 epicatechin; 7 syringic acid; 8 syringaldehyde; 9 ferulic acid; 10 ferulic acid; 11 rutin; 12 ellagic acid; 13 myricetin; 14 quercetin; 15 juglone.
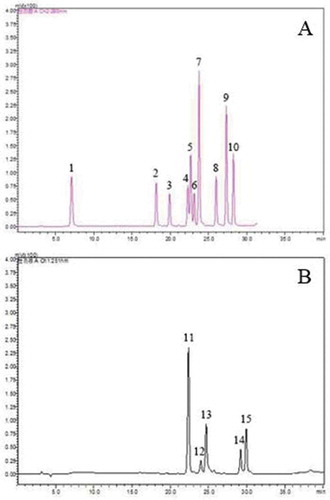
Figure 4. Antioxidant activity changes of walnut green husks during different stages of development. Results represent the means ± standard deviation (n = 3). Different little letter near the bar indicate significantly difference at p < 0.05 level. DPPH: 1,1-diphe-nyl-2-picrylhydrazyl, FRAP: Ferric reducing ability of plasma, ABTS: 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid). IC50 means the sample concentration providing 50% inhibition.
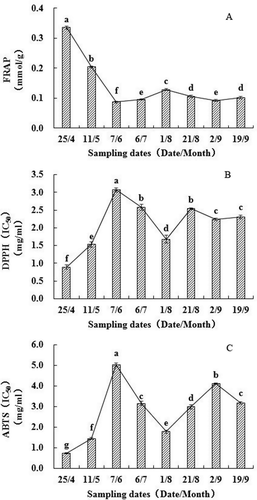
Table 2. Correlation coefficient between antioxidant activity and phenolics content.

