Figures & data
Figure 1. Amount and standard deviation of DNA extracted from six Tunisian brands of turkey salami. (A.1) Bardakci and Skibinski2 Standard Protocol, (A.2) with homogenization by a ball mill (vibro mill MM 400).
1DNA yield is calculated by multiplying the DNA concentration measured by nanodrop (ng/µl) by the elution volume (µl) divided by the sample mass (mg). 2Phenol Chloroform Isoamyl alcohol protocol corresponding to Bardakci and Skibinski protocol.
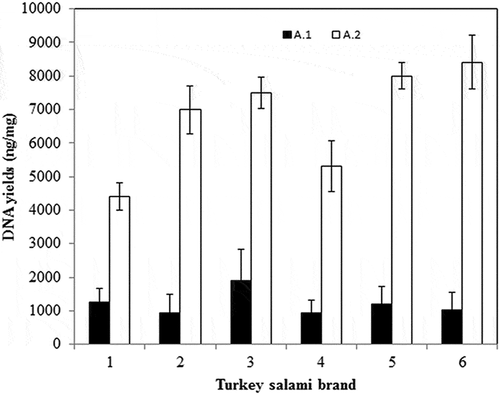
Figure 2. DNA yield1 and purity (A260/280) obtained from six Tunisian brands of turkey salami using NaOH extraction protocol (Protocol 1), Phenol Chloroform Isoamyl protocol2 (using 3.5 ml of the acqueous phase) (Protocol 2), Phenol Chloroform Isoamyl protocol2 (using the rest of the acqueous phase) (Protocol 3), CTAB protocol (Protocol 4), Chloroform protocol (Protocol 5).
1DNA yield is calculated by multiplying the DNA concentration measured by nanodrop (ng/µl) by the elution volume (µl) divided by the amount of starting material (mg). 2Phenol Chloroform Isoamyl alcohol protocol corresponding to Bardakci and Skibinski protocol with homogenization by a ball mill (vibro mill MM 400). Median values are indicated by the solid line within each box, and the box extends to upper and lower quartile values. The dashed line represents the median of the different DNA yields obtained for all tests for all Tunisian turkey salami types.
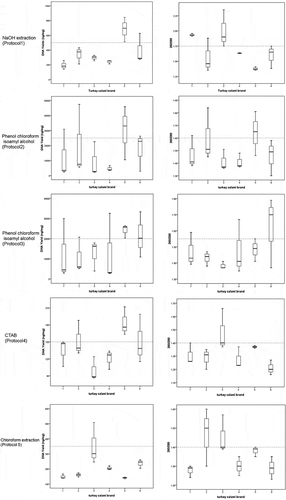
Figure 3. Agarose gel electrophoresis of total DNA isolated from brand 2 of Tunisian turkey salami using Bardakchi and Skibinski1 with homogenization using only 3.5 ml of acqueous phase (Protocol 2) (lane 1), Bardakchi and Skibinski1 with homogenization using the rest of the acqueous phase (Protocol 3) (lane 2), NaOH extraction protocol (Protocol 1) (lane 3), CTAB method (Protocol 4) (lane 4), Choloroform extraction method (Protocol 5) (lane 5) and DNeasy blood and tissue kit (Protocol 6) (lane 6). Lane 7: DNA KB-Ladder includes 9 fragments ranging from 0.5 to10 kilobases (kb) (BioBasic Inc). (a) using the same volume of 10 µl for all DNA extract; b: using the same concentration of 200 ng for all DNA extract
1Phenol Chloroform Isoamyl alcohol protocol corresponding to Bardakci and Skibinski protocol.
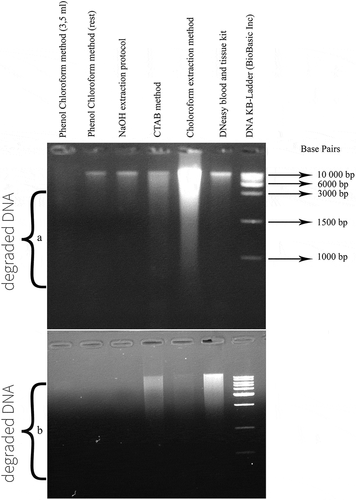
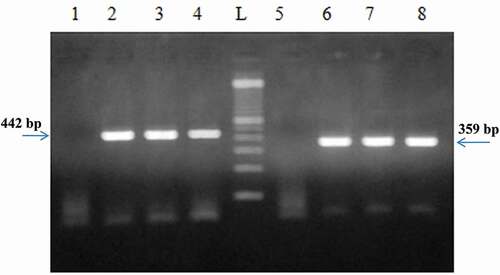
Figure 4. (a) PCR product generated by D-loop primers amplification of DNA extracted from chicken and turkey peripheral blood. (b) PCR product generated by cytochrome b primers amplification of DNA extracted from chicken and turkey peripheral blood.
P1: negative control of primers, P2: 100% chicken (45 ng), P3: 100% turkey (45 ng), P4: 10% chicken and 90% turkey, P5: 5% chicken and 95% turkey, P6: 2% chicken and 98% turkey, P7: 1% chicken and 99% turkey, L: 100 bp DNA ladder (Catalog Number: 15628019; ThermoFisher Scientific)
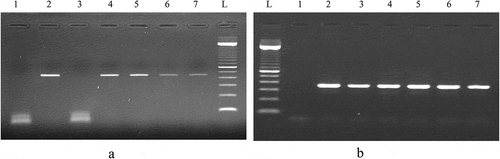
Table 1. DNA yields (ng DNA/mg sample), A260/A280 and A260/A230 Ratios of DNA extracted from 6 brands of Tunisian turkey salami with five DNA extraction methods using an amount of 50 mg of starting material. The mean value and standard deviation are shown for each sample.
Figure 5. (a) PCR product generated by D-loop and cytochromeb primers amplification of DNA extracted from brand 2 of turkey salami using 50 mg as an amount of starting material: P1→P5: D-loop primers. P6→P10: Cytochrome b primers. L:100 bp DNA ladder (Catalog Number:15628019; ThermoFisher Scientific). P1: negative control of D-loop primers. P6: negative control of cytochrome b primers, P2 and P7: NaOH extraction protocol (Protocol 1), P3 and P8: Phenol Chloroform Isoamyl alcohol extraction protocol1 (using 3.5 ml of the acqueous phase) (Protocol 2), P4 and P9: Phenol Chloroform Isoamyl alcohol extraction protocol1 (using the rest of the acqueous phase) (Protocol 3), P5 and P10: DNeasy blood and tissue kit (Protocol 6). (b) Turkey salami heteroplasmy exploration, cytochrome b sequence validation: turkey salami, chicken reference and turkey reference.
1Phenol Chloroform Isoamyl alcohol protocol corresponding to Bardakci and Skibinski protocol with homogenization by a ball mill (vibro mill MM 400).
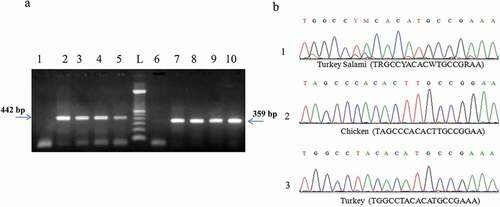
Table 2. DNA Yields (ng DNA/mg sample), A260/A280, A260/A230 Ratios and results of PCR using cytochrome b and D-loop primers of DNA extracted from brand 2 of Tunisian turkey salami with the Phenol Chloroform Isoamyl Alcohol protocol using different amount of starting material (2, 5, 7, 10 and 25 mg). The Mean Value and Standard Deviation are shown for each sample.
Figure 6. (a) Histogram of DNA yields1 obtained from brand 2 of Tunisian turkey salami using four extraction protocols (A) NaOH extraction protocol (Protocol 1) (B) Phenol Chloroform Isoamyl alcohol extraction protocol2 (using 3.5 ml of the acqueous phase) (Protocol 2) (C) Phenol Chloroform Isoamyl alcohol extraction protocol2 (using the rest of the acqueous phase) (Protocol 3) (D) DNeasy blood and tissue kit (Protocol 6) using differents masses of samples (2, 5, 7, 10 and 25 mg). (b) and (c) PCR product generated by D-loop and cytochrome b primers amplification of DNA extracted from brand 2 of turkey salami using different masses of starting material. (b): NaOH extraction protocol (Protocol 1), (c): DNeasy blood and tissue kit (Protocol 6): 2 mg (Lane 2 and 8), 5 mg (Lane 3 and 9), 7 mg (Lane 4 and 10), 10 mg (Lane 5 and 11) and 25 mg (Lane 6 and 12). P1→P6: D-loop primers, P7→P12: cytochrome b primers, L: 100 bp DNA ladder (Catalog Number:15628019; ThermoFisher Scientific). P1: negative control of D-loop primers, P7: negative control of cytochrome b primers.
1DNA yield is calculated by multiplying the DNA concentration measured by nanodrop (ng/µl) by the elution volume (µl) divided by the sample mass (mg). 2Phenol Chloroform Isoamyl alcohol protocol corresponding to Bardakci and Skibinski protocol with homogenization by a ball mill (vibro mill MM 400).
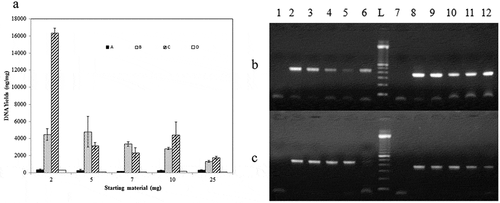
Figure 7. PCR product generated by D-loop and cytochrome b primers amplification of DNA extracted from brand 2 of turkey salami from swabs using NaOH extraction protocol and Phenol Chloroform Isoamyl alcohol protocol. P1→P4: D-loop primers, P5→P8: Cytochrome b primers, P1: negative control of D-loop primers protocol corresponding to Bardakci and Skibinski protocol with homogenization by a ball mill (vibro mill MM 400)., P5: negative control of cytochrome b primers, P2 and P6: NaOH extraction protocol (Protocol 1), P3 and P7: Phenol Choloroform Isoamyl alcohol extraction protocol1 (using 3.5 ml of the acqueous phase) (Protocol 2), P4 and P8: Phenol Choloroform Isoamyl alcohol extraction protocol1 (using the rest of the acqueous phase) (Protocol 3), L: 100 bp DNA ladder (Catalog Number: 15628019; thermoFisher Scientific).
1Phenol Chloroform Isoamyl alcohol protocol corresponding to Bardakci and Skibinski protocol with homogenization by a ball mill (vibro mill MM 400).