Figures & data
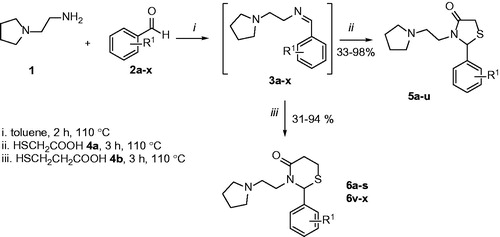
Figure 1. Similarity at chemical structure of acetylcholine, thiazolidin-4-ones (5) and thiazinan-4-ones (6).
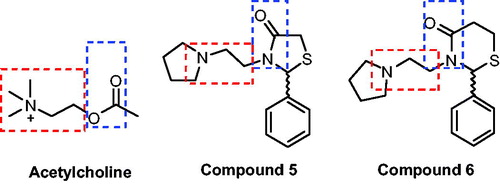
Figure 2. In vitro effect of compounds 5j, 6a, 6 h, 6j, 6k and 6n on the activity of AChE in cerebral cortex of rats. *p < .05, **p < .01, ***p < .001 when compared to control group (water or MeOH).
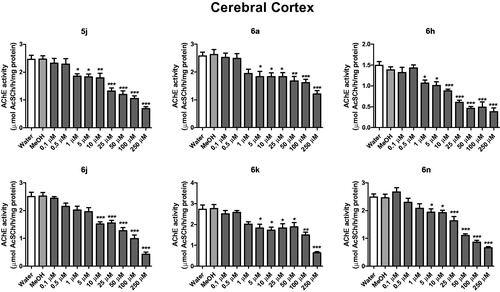
Figure 3. In vitro effect of compounds 5j, 6a, 6 h, 6j, 6k and 6n on the activity of AChE in cerebral hippocampus of rats. *p < .05, **p < .01, ***p < .001 when compared to control group (water or MeOH).
Figure 4. In vitro cytotoxicity activity of compounds 5j, 6a, 6 h, 6j, 6k and 6n to cell viability of the primary astrocyte culture at 100 μM, after for 48 h (A) and 72 h (B) the treatment.
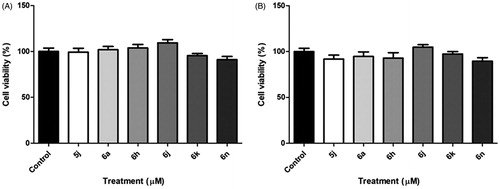
Table 1. IC50 of AChE inhibition for thiazolidin-4-ones 5 and thiazinan-4-ones 6.