Figures & data
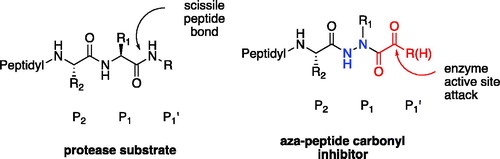
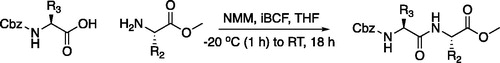
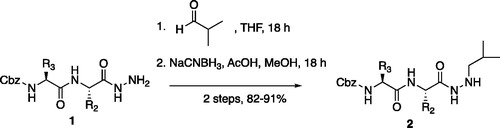
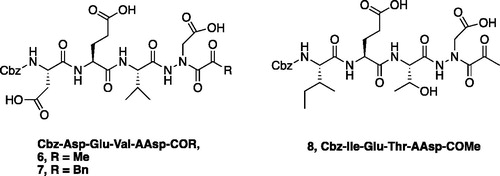
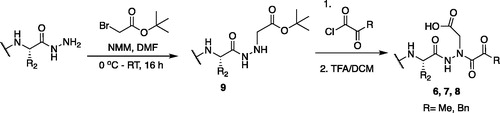
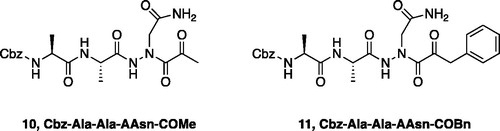
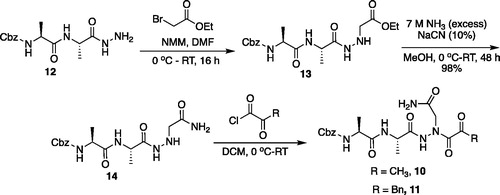
Figure 4. (A) Coupling procedure for aza-peptide aldehyde warhead. (B) Coupling procedure for aza-peptide ketone warhead. (C) Library of synthesised aza-peptide aldehyde and ketone inhibitors for the proteasome.
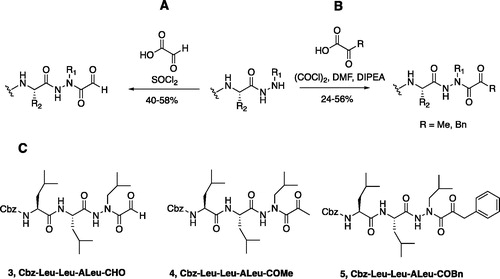
Table 1. Inhibition of β5 active site of the human 20S proteasome by aza-peptide aldehydes and ketones
Table 2. Inhibition of human caspase-3 and caspase-6 by aza-peptide ketones
Table 3. Inhibition of S. mansoni and I. ricinus legumains by aza-peptide ketones
Table 4. Cross-reactivity of human cathepsin B and bovine pancreas α-chymotrypsin with aza-peptide aldehydes and ketones
Figure 9. Caspase-3 in complex with Cbz-Asp-Glu-Val-AAsp-COMe (Compound 6). Compound 6 is observed residing in the active site of caspase-3 at a resolution of 2.73 Å after thiohemiacetal covalent-bond formation to the methyl ketone warhead of 6.
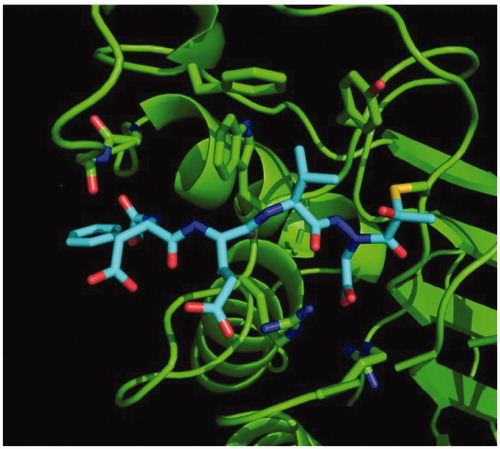
Figure 10. Mechanism of inhibition of caspase-3 by the aza-peptide methyl ketone inhibitor Cbz-Asp-Glu-Val-AAsp-COMe (Compound 6). The inhibitor ketone carbonyl carbon is the site of nucleophilic addition by the active-site Cys163 sulphur atom, resulting in covalent bond formation.
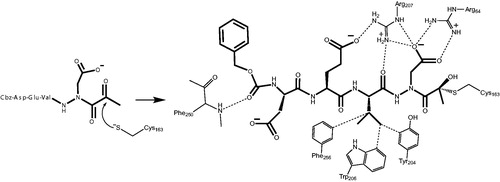
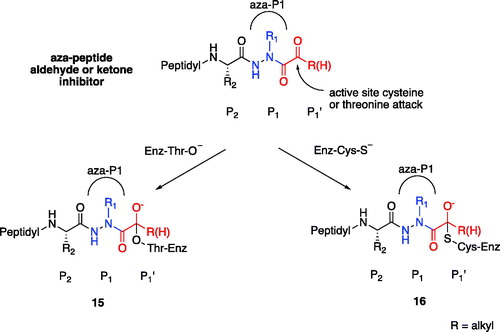
Figure 11. Proposed mechanism of inhibition by aza-peptide aldehydes and ketones reacting with (a) the proteasome and with (b) other clan CD cysteine proteases. The carbonyl carbon is expected to be the site of attack by nucleophilic residues: a Thr-O with the proteasome and a Cys-S with clan CD cysteine proteases.