Figures & data
Scheme 1. Synthetic route of compounds 1. Reagents and conditions: (i) BnCl, NaOH, MeOH/H2O, 70 °C, 6 h, 80% yield; (ii) appropriate amines, EtOH, reflux, overnight, (iii) MnO2, 1,4-dioxane, (iv) Br2, CHCl3, rt, 10 min; (v) PPh3, CH2Cl2, 30 min, (vi) tert-BuOK, dry THF, (vii) BBr3, DCM, 0 °C to rt, 2 h, 55–72% yield.
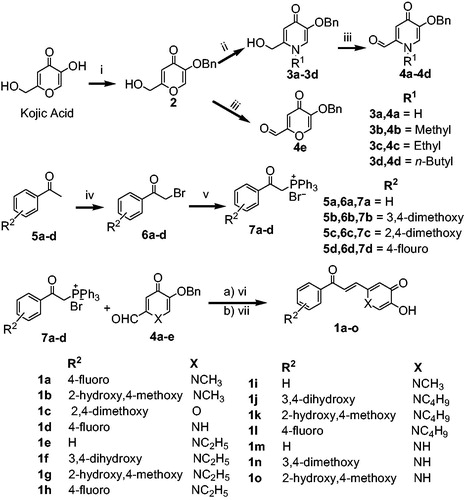
Figure 2. Inhibitory effect of 1a, 1d, 1f, and 1n on the monophenolase activity of mushroom tyrosinase. The assays were performed at 30 °C and pH 6.8.
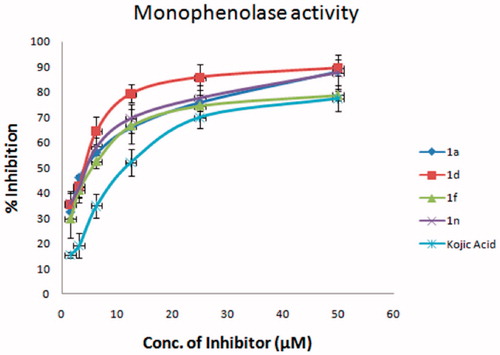
Table 1. Inhibition of compounds (1a–1o) (50 µM) on monophenolase activity of mushroom tyrosinase under the conditions of 30 °C and pH 6.8.
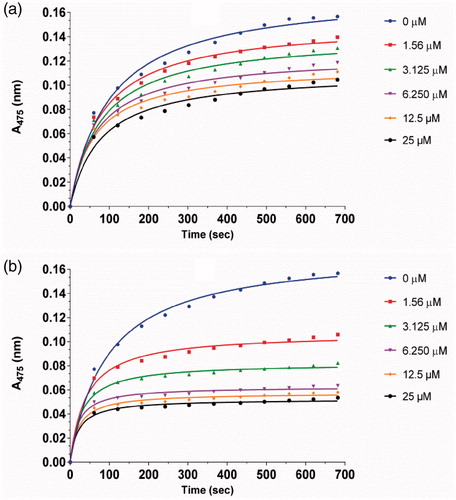
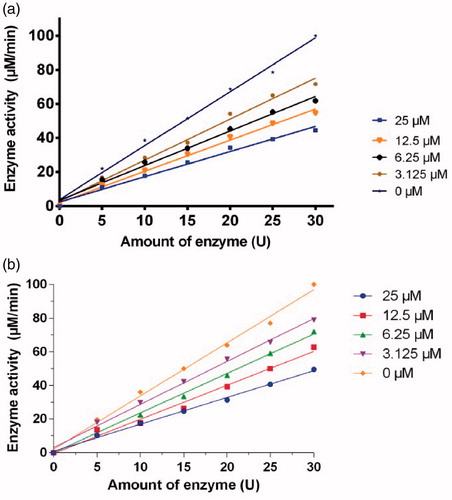
Figure 3. Inhibitory effect of different concentrations of 1a (a) and 1d (b) on the diphenolase activity of tyrosinase. The assays were performed at 30 °C and pH 6.8.