Figures & data
Figure 1. Representative gall types induced by the Psylloidea. (a) leaf-roll gall induced by Trioza alacris on Laurus nobilis in Europe (source: Plant Parasites of Europe; bladmineerders.nl/parasites/animalia/arthropoda/insecta/hemiptera/ sternorrhyncha/psylloidea/triozidae/trioza/trioza-alacris/ (accessed on May 20, 2021). (b): pit gall induced by Trioza ocoteae on Ocotea acutifolia in South America (source: Lizer Trelles and Molle Citation1945), (c) urn shaped galls induced by Schedotrioza multidunea on a species of Eucalyptus in Australia (source: Houard Citation1923), (d) closed pouch galls induced by Pachypsylla celtidismamma on Celtis occidentalis in North America (source: Riley Citation1881), (e) and spherical, closed galls as in Pauropsylla udei on a species of Ficus in Indonesian islands (Rübsaamen Citation1899), (f) spherical gall induced by Schedotrioza eucalypti on Eucalyptus dives in Australia (inset–dehisced gall) (bar = 5 mm) (source: unpublished work by authors); (g) spherical closed galls (with sugary accumulation) induced by Glycaspis (Synglycaspis) sp. A on E. macrorhyncha in Australia (bar = 5 mm); the gall on left has been slit open with a razor blade to expose the sugary filaments (source: Sharma et al. Citation2015a). (Scale bars not available for Figures a–e in original texts).
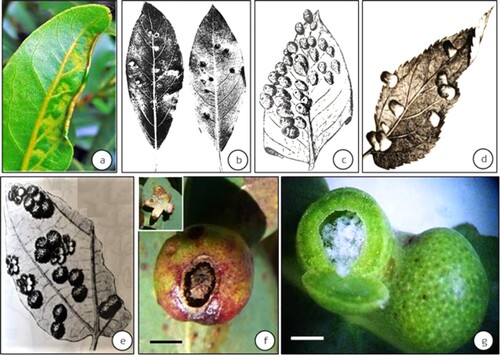
Figure 2. Examples of complex galls induced by the Psylloidea from the Indian subcontinent. (a) fir-cone like galls on Mangifera indica induced by Apsylla cistellata (source: Raman et al. Citation2009 Tropical Zoology) (inset–vertical-sectional view of a gall) (bar = 4 cm), (b) Two-tier saccular galls induced by Phacopteron lentiginosum on Garuga pinnata (courtesy: M. Nasser, University of Calicut, Kerala, India). Inset: Vertically slit gall showing the upper tier of the saccular gall and the supporting lower tier of collar-like growth. A late-stage immature can be seen at 1 o’clock position in the sac-part of this two-tier gall (bar = 5 cm).

Figure 3. Lerp-constructing Psylloidea. (a) Lerps of the III (α), IV (β), and V (γ) stage immatures [bar = 1 mm], (b) Enlargement of a lerp showing sugary filaments constructed by early immature stages of an unnamed species of Glycaspis on Eucalyptus sideroxylon in Australia [bar = 100 μm], (c) The same Glycaspis (male) adult in Australia [bar = 1 mm] (Source: Sharma et al. Citation2013).
![Figure 3. Lerp-constructing Psylloidea. (a) Lerps of the III (α), IV (β), and V (γ) stage immatures [bar = 1 mm], (b) Enlargement of a lerp showing sugary filaments constructed by early immature stages of an unnamed species of Glycaspis on Eucalyptus sideroxylon in Australia [bar = 100 μm], (c) The same Glycaspis (male) adult in Australia [bar = 1 mm] (Source: Sharma et al. Citation2013).](/cms/asset/c5790a71-9359-468f-acb7-dc510d260a58/tjpi_a_2065371_f0003_oc.jpg)
Figure 4. Developmental sequence of the two-tier saccular gall induced by Phacopteron lentiginousm on the leaves of Garuga pinnata: from oviposition to dehiscence (not to scale). Arrow pairs: meristematic activity; (●): immature. (source: Raman Citation2011). ‘e’ is egg and part of egg stalk is buried in the leaf tissue.
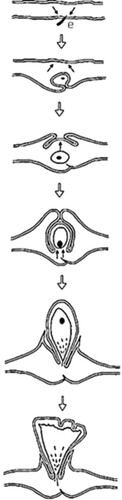
Figure 5. Sensilla on the mouthparts of an unnamed species of Glycaspis on Eucalyptus sideroxylon (sensilla on extended labium, lb; stylet bundle, sb) [bar = 1 μm] (Source: Sharma et al. Citation2013).
![Figure 5. Sensilla on the mouthparts of an unnamed species of Glycaspis on Eucalyptus sideroxylon (sensilla on extended labium, lb; stylet bundle, sb) [bar = 1 μm] (Source: Sharma et al. Citation2013).](/cms/asset/0325ce12-6626-4988-a59a-b607c3b7736c/tjpi_a_2065371_f0005_ob.jpg)
Figure 6. (a) Camera-lucida drawing of a portion of the nymphal bed of an early immature of the gall-inducing Glycaspis (Synglycaspis) sp. A, on Eucalyptus macrorhyncha. (nutritive cells, nc; vascular trace, vt; stylet track, st; stomatal aperture, s; proliferating epidermal cells, pe; neoformed meristem, nm; dividing cells, circled area) [bar = 100 μm]. (b) submicroscopic changes in E. macrorhyncha leaf (cross-sectional view) due to feeding by Glycaspis (Synglycaspis) A: (endosome, es; chloroplast, ch; secondary metabolic inclusion, sm; autophagic vacuole, av; cell junctions, cj; mitochondrion, m; desmosomal condensation, dc; oil inclusion, oi; dissolved middle lamella, ⇔; stylet track, circled area) [bar = 1 μm] (Source: Sharma et al. Citation2015a).
![Figure 6. (a) Camera-lucida drawing of a portion of the nymphal bed of an early immature of the gall-inducing Glycaspis (Synglycaspis) sp. A, on Eucalyptus macrorhyncha. (nutritive cells, nc; vascular trace, vt; stylet track, st; stomatal aperture, s; proliferating epidermal cells, pe; neoformed meristem, nm; dividing cells, circled area) [bar = 100 μm]. (b) submicroscopic changes in E. macrorhyncha leaf (cross-sectional view) due to feeding by Glycaspis (Synglycaspis) A: (endosome, es; chloroplast, ch; secondary metabolic inclusion, sm; autophagic vacuole, av; cell junctions, cj; mitochondrion, m; desmosomal condensation, dc; oil inclusion, oi; dissolved middle lamella, ⇔; stylet track, circled area) [bar = 1 μm] (Source: Sharma et al. Citation2015a).](/cms/asset/f112c2a9-6a4f-4743-912d-6ea109b0fa7b/tjpi_a_2065371_f0006_ob.jpg)
Figure 7. Isotopic carbon and nitrogen values in the normal and gall-bearing leaves of Eucalyptus macrorhyncha hosting Glycaspis (Synglycaspis) sp. A in Australia. (a) δ13C values in uninfested (U) and infested [1C–5C: five developmental stages of Glycaspis (Synglycaspis)]. (b) δ15N values in uninfested (U) and infested [1N–5N: five developmental stages of Glycaspis (Synglycaspis)]. Error bars are shown along the y axes of the graphs (source: Sharma et al. Citation2015c).
![Figure 7. Isotopic carbon and nitrogen values in the normal and gall-bearing leaves of Eucalyptus macrorhyncha hosting Glycaspis (Synglycaspis) sp. A in Australia. (a) δ13C values in uninfested (U) and infested [1C–5C: five developmental stages of Glycaspis (Synglycaspis)]. (b) δ15N values in uninfested (U) and infested [1N–5N: five developmental stages of Glycaspis (Synglycaspis)]. Error bars are shown along the y axes of the graphs (source: Sharma et al. Citation2015c).](/cms/asset/af920c24-ed31-45b5-bdaf-8cc78837aaf6/tjpi_a_2065371_f0007_ob.jpg)
Figure 8. Galls modifying the vegetative shoot buds of Juncus articulatus (Juncaceae) induced by Livia junci distributed in Continental Europe (sensu Epstein Citation2014). Gall development inhibits leaf expansion and the elongation of internodal segments. (Source: Jiří Kameníček: Biolib.cz) (b) A line sketch of the gall (Source: Schmidt and Meyer Citation1966). (No scale bars are indicated by respective authors).
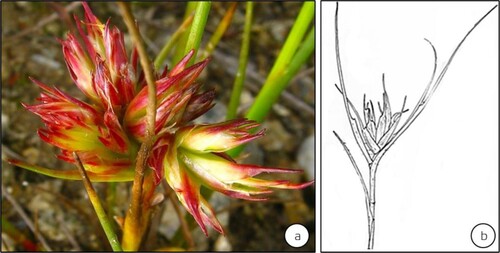
Figure 9. Antennae on adult female of gall-inducing Glycaspis (Synglycaspis) sp. A that specifically oviposit on E. macrorhyncha leaves. (a) close up of the antennae (trichoid sensilla on adult antenna, ts) [bar = 50 μm]. (b) close up of cupola shaped rhinarium (basiconic sensillum, bs; rhinarium, rh) [bar = 10 μm]. (Source: Sharma et al. Citation2015a).
![Figure 9. Antennae on adult female of gall-inducing Glycaspis (Synglycaspis) sp. A that specifically oviposit on E. macrorhyncha leaves. (a) close up of the antennae (trichoid sensilla on adult antenna, ts) [bar = 50 μm]. (b) close up of cupola shaped rhinarium (basiconic sensillum, bs; rhinarium, rh) [bar = 10 μm]. (Source: Sharma et al. Citation2015a).](/cms/asset/393b4701-b9b2-426d-ba54-2cdce6a85869/tjpi_a_2065371_f0009_ob.jpg)
Figure 10. Sterols in young (▪) and mature (
 ) predominant in young leaves of E. macrorhyncha, is the potential sterol that regulates the specificity of the Glycaspis (Synglycaspis) sp. A. x-axis – molecular weights of sterols; y-axis – mole % of sterols. Vertical bar represents the SE value. (Source: Sharma et al. Citation2016).
) predominant in young leaves of E. macrorhyncha, is the potential sterol that regulates the specificity of the Glycaspis (Synglycaspis) sp. A. x-axis – molecular weights of sterols; y-axis – mole % of sterols. Vertical bar represents the SE value. (Source: Sharma et al. Citation2016).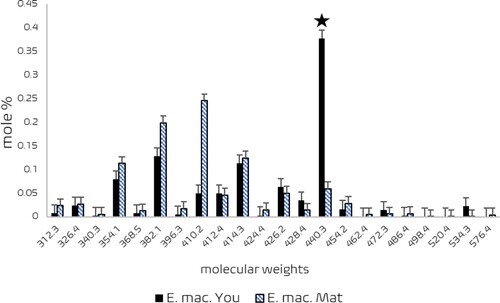
Table 1. Sterols in normal and developing stages of galls induced by Glycaspis (Synglycaspis) sp. A on the leaves of Eucalyptus macrorhyncha (adapted from Sharma et al. Citation2015c).
