Figures & data
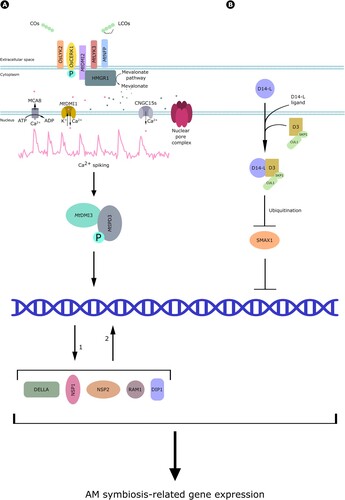
Figure 1. Classification and chemical structure of Strigolactones. These are generally composed of a tricyclic lactone (ABC) connected, by an enol-ether bond, to a butenolide moiety (red), which represents the most conserved trait of the molecule between plant taxa.
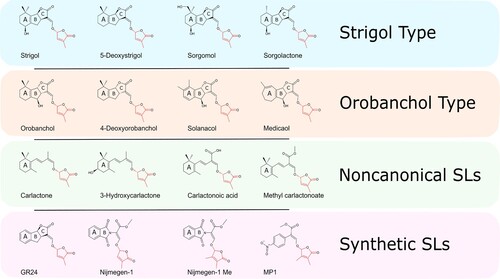
Figure 2. General scheme of Strigolactone biosynthesis. Strigolactones are synthesized from trans-β-carotene and the large diversity of SL structures is obtained thanks to the recruitment of different enzymes in different plant species.
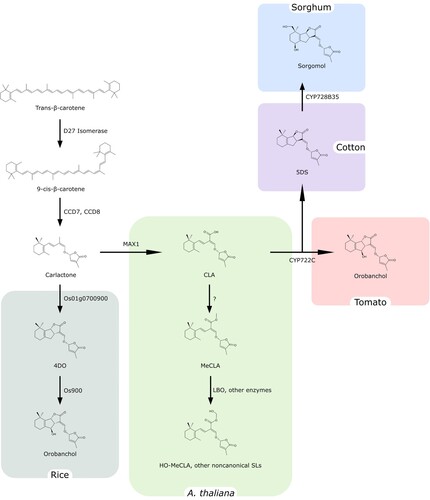
Figure 3. Perception of Strigolactones and signaling. Strigolactones are perceived by D14, which forms a complex with D3 and D53, a negative regulator of downstream signaling. The molecule is then hydrolyzed to hydroxymethylbutenolide (HMB) and an ABC ring formyltricyclic lactone (ABC-FTL), while D14 and D53 are degraded, allowing the activation of downstream signaling.
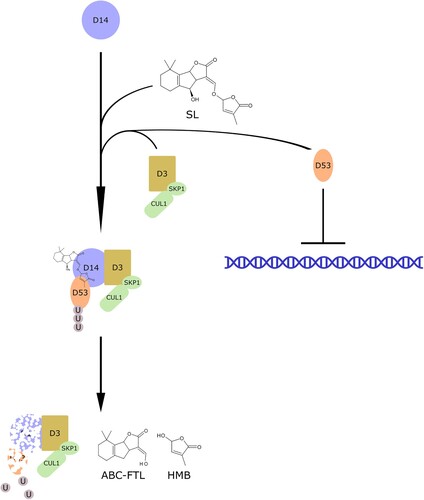
Figure 4. Chemical structure of chitin-derived fungal signals. Myc-factors characterized in arbuscular mycorrhizal fungi include (A) tetra-chito-oligosaccharide, composed of four monomers of N-acetylglucosamine (GlcNAc), and (B) tetra-lipo-chito-oligosaccharide bearing a 16:1 fatty acid chain (red) and a carbamoyl group (blue). (C) octa-chito-oligosaccharide (CO8) are common elicitors of defense responses.
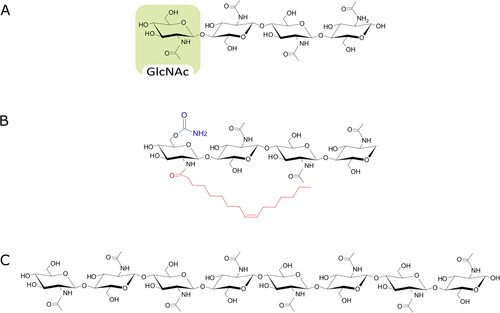
Figure 5. Fungal biosynthesis of chito-oligosaccharides. N-acetylglucosamine (GlcNAc) monomers are transferred from UDP-GlcNAc to the forming chitin chain by the action of the glycosyltransferase and the chitin synthase (yellow). Chitin chains extruded in the extracellular space through a channel composed of chitin synthase transmembrane domains can then be cleaved by several chitinases (Chit33, Chit42) to produce chito-oligosaccharides of different length. Finally, a deacetylase can further remove acetyl groups from the newly formed oligomers. Purple = UDP; green = GlcNAc monomers; orange = Glc monomers.
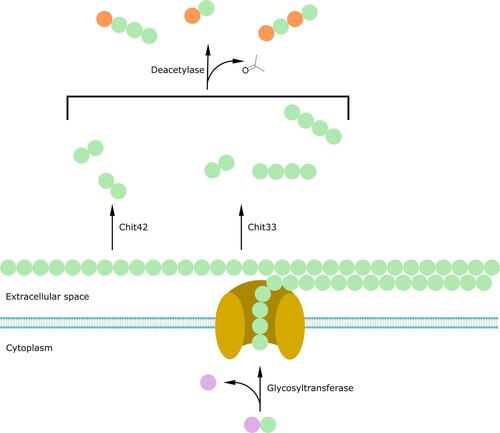
Figure 6. Myc-factor perception. (A) Studies in rice demonstrated that COs are perceived by OsLYK2 and OsCERK1, which is activated by phosphorylation. Investigations in M. truncatula showed that CO and LCO perception requires the action of DMI2, LYK3 and NFP. DMI2 binds to HMGR1, which starts mevalonate production and downstream signaling. This includes nuclear Ca2+ spiking, which also requires the activity of a few nuclear envelope-localized proteins: the cationic channel DMI1, the cyclic nucleotide-gated Ca2+ channel CNGC15s, the MCA8 Ca2+ pump and several nucleoporins in the nuclear pore complex. Ca2+ spiking is further decoded by the calcium and calmodulin-dependent kinase DMI3, which phosphorylates the transcriptional regulator MtIPD3, activating in turn DELLA, DIP1, NSP2, NSP1 and RAM1 transcription factors and driving the expression of AM-related genes. (B) Perception of alternative AM fungal signals by D14-L. After the perception of an unidentified ligand, D14-L form a complex with D3, driving the ubiquitination of the negative regulator SMAX1 and thus allowing downstream signaling culminating in gene regulation and symbiotic plant responses.