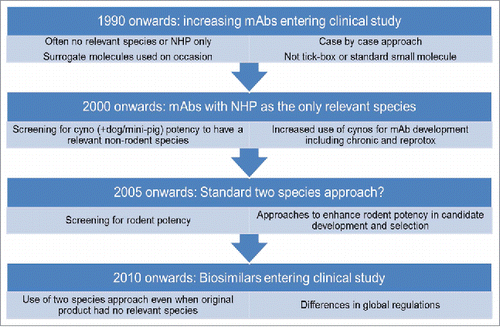
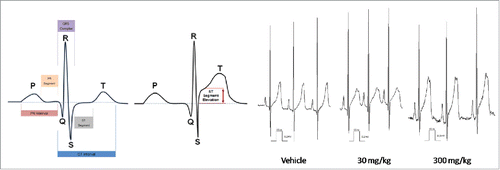
Figures & data
Table 1. Questions for case studies addressed during the breakout sessions.
Table 2. In vivo studies for anti-ADAMTS-5.
Table 3. In vitro studies for anti-ADAMTS-5.
Table 4. In vivo studies for anti-DLL4 IgG1 mAb.
Table 5. In vivo studies for anti-DLL4 Fab’2 fragment.
Table 6. In vivo studies for anti-amyloid β mAb.
Table 7. In vitro studies for anti-amyloid β mAb.
Table 8. Summary of case-study data and the ability of existing in vitro and in silico technologies to detect or predict toxicities observed in the in vivo studies.