Figures & data
Figure 1. Identifying and characterizing MerTK agonist antibodies for engineering anti-CD20/MerTK bispecific antibodies capable of inducing phagocytosis of B cells by macrophages (a) Characterization of lead MerTK agonist antibodies measured by their ability to induce pAKT (Ser-473) in human MerTK+ macrophages using HTRF assay (Mean ± S.D of 2 technical replicates). Gas6-Fc is a positive control for MerTK activation. (b) Schematic depiction of B cell-dependent engagement of MerTK via anti-CD20/MerTK bispecific antibody. (c) Imagestream analysis of phagocytosis of live Raji cells by human primary macrophages in the presence of indicated bispecific antibody. A representation of Imagestream analysis is shown. Quantification is also presented (Mean ± S.D of one replicate each from 3 donors). (d-f) Flow cytometric analysis of phagocytosis of live Raji cells by human primary macrophages in the presence of indicated bispecific antibody, inhibitors and blocking agents (Mean ± S.D of one replicate each from 3 donors) (g) Activation of MerTK (phosphotyrosine) by the bispecific antibody in the presence of target Raji cells. Results are from using macrophages derived from two different human donors. See Supplementary Figure 5 for extended western blot. (h-i) TNF and IL-6 production by human primary macrophages stimulated with indicated plate coated antibodies. (Mean ± S.D of one replicate each from three different donors)
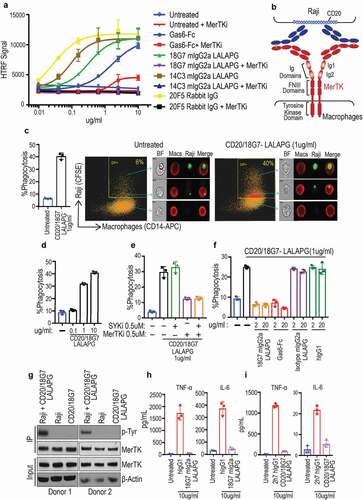
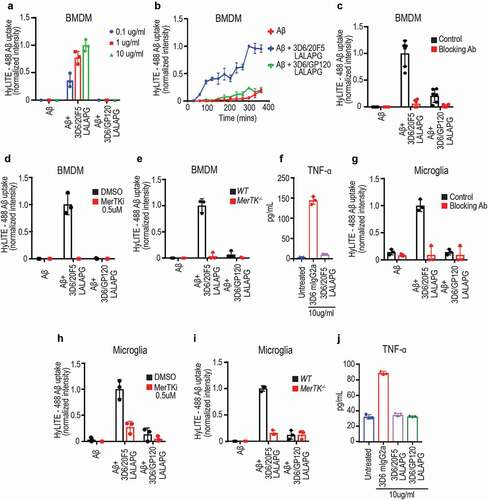
Figure 2. Anti-Aβ/MerTK bispecific antibody induces uptake of aggregated Aβ by BMDMs or microglia (a) Antibody dose-dependent uptake of aggregated Aβ by mouse BMDMs (Mean ± S.D of 3 technical replicates). (b) Time course of aggregated Aβ uptake by mouse BMDMs (Mean ± S.D of 3 technical replicates). Aggregated Aβ at 1 μM with antibodies at 10 ug/ml concentration. (c-e) BMDM uptake of aggregated Aβ induced by anti-Aβ/MerTK bispecific antibody is MerTK-dependent, as demonstrated by competition with the parental anti-MerTK antibody (20F5 Rabbit IgG) (c), inhibition by a MerTK SMI (d), or loss of activity in MerTK −/- BMDMs (e) (Mean ± S.D of 3 technical replicates). Aggregated Aβ at 1 μM, and antibodies at 10 ug/ml concentration. (f) TNF production by BMDMs stimulated with 3D6 mIgG2a (FcγR agonist) or 3D6/20F5 mIgG2a-LALAPG (MerTK agonist) (Mean ± S.D of 3 technical replicates). (g-i) Microglial cell uptake of aggregated Aβ induced by anti-Aβ/MerTK bispecific antibody is MerTK-dependent, as demonstrated by competition with the parental anti-MerTK antibody (20F5 Rabbit IgG) (g), inhibition by a MerTK SMI (h), or loss of activity in MerTK −/- microglia (i) (Mean ± S.D of 3 technical replicates). (j) TNF production by microglia stimulated with 3D6 mIgG2a (FcγR agonist) and 3D6/20F5 mIgG2a-LALAPG (MerTK agonist) and 3D6/GP120 mIgG2a-LALAPG (control) (Mean ± S.D of 3 technical replicates)