Figures & data
Figure 1. (a) Cartoon representation of monospecific and bispecific antibody formats investigated. The isotype for the anti-IL-17 antibody is IgG1, whereas the anti-IL-13 and BsIgG antibodies are both IgG4. All antibodies have κ light chains. Half-antibodies containing knobs-into-holes mutationsCitation34 (‘knob’ mutation (T366W) in the anti-IL-13 arm or ‘hole’ mutations (T366S:L368A:Y407V) in the anti-IL-17arm) were expressed and purified separately before assembly in vitro as described previously.Citation35,Citation36 A hinge stabilizing mutation (S228P) was introduced into the IgG4 to attenuate Fab arm exchange.Citation37,Citation38 (b) Viscosity of fragments (F(ab’)2 and Fab) or full-length anti-IL-13/IL-17 bispecific or its parent antibodies are shown. (c) The effect of 150 mM NaCl or 150 mM Arg-HCl on solution viscosity is shown. The dotted line represents the viscosity of parent IgG antibody level as a reference. Data shown are the mean values (n = 3, ± SD) measured by rheometer at 23ºC and 150 mg/mL total protein concentration in 20 mM His-HCl pH 6.0 (buffer) in the absence or presence of excipients
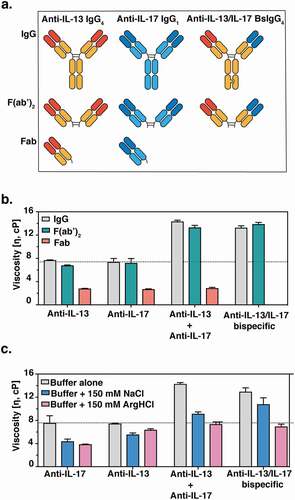
Figure 2. X-ray crystallographic structures of anti-IL-13 (lebrikizumab)Citation40 and anti-IL-17 (MCAF5352ACitation41) antibody Fabs complexed with their respective ligands displaying aromatic residues chosen for mutational studies. (a) View of anti-IL-13/IL-13 interface depicting important viscosity reducing residues (PDB 4I77).Citation40 The anti-IL13 VH is cyan and VL is light pink, with dark cyan and magenta side chains within 4 Å of IL-13. IL-13 is gray with yellow epitope within 4 Å of anti-IL-13. (b) View of anti-IL-17/IL-17F interface depicting important residues (PDB 6PPG). Anti-IL-17F VH is cyan and VL is light pink, with dark cyan and magenta side chains within 4 Å of IL-17. IL-17F is gray with yellow epitope within 4 Å of anti-IL-17F. See the Supplementary Materials for the sequences for the variable domains for the anti-IL-13 (Figure S6) and anti-IL17 (Figure S7) antibodies
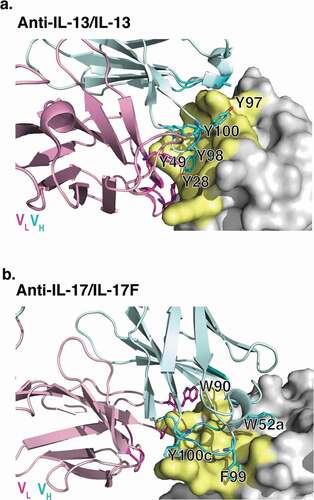
Figure 3. MD simulations of (a) anti-IL-13 (lebrikizumabCitation40) and (b) anti-IL-17 (MCAF5352ACitation41) variable heavy (VH) and variable light (VL) domains in the presence of arginine. Data shown are the mean number of arginine contacts from 20 independent simulations. Residues that show >2σ or >3σ deviations from the mean (μ) number of arginine contact number of all residues in chains are highlighted. Kabat numbering is used.Citation42.
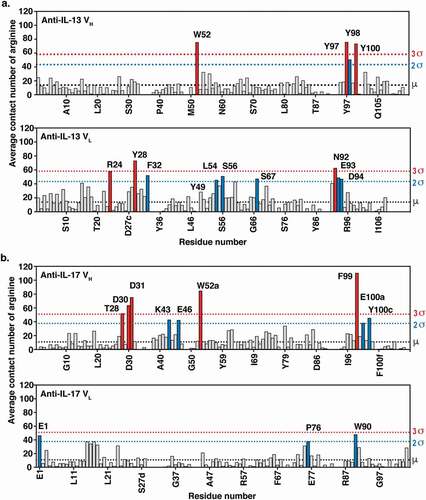
Figure 4. Viscosity of antibody variants. Monospecific anti-IL-13 IgG4 and anti-IL-17 IgG1 antibody variants were mixed with the parent anti-IL-17 IgG1 and anti-IL-13 IgG4 antibody counterparts, respectively, at a 1:1 ratio and a final total concentration of 150 mg/mL. The viscosity of the solutions was then measured by rheometer at 23ºC. (a) Viscosity of mixtures of anti-IL-13 charged variants and the anti-IL-17 parent antibody. (b) Viscosity of mixtures of anti-IL-17 charged variants and the anti-IL-13 parent antibody. (c) Viscosity of mixtures of anti-IL-13 and anti-IL-17 aromatic variants with their parent IgG antibody counterpart. (d) Viscosity of anti-IL-13/IL-17 BsIgG4 variants at 150 mg/mL final concentration. Data shown are the mean values (n = 2, ± SD) measured in 20 mM His-HCl buffer at pH 6.0. The dotted line shows the parent antibody viscosity level as a reference
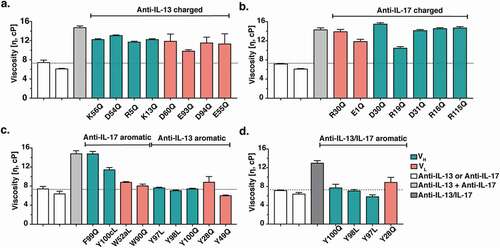
Table 1. Antigen-binding kinetics of anti-IL-13 antibody variants. Binding kinetics of anti-IL-13 variants for IL-13 were measured by SPR at 37°C, pH 7.4 and data were fitted to a 1:1 binding model. *Variants that reduced viscosity and have comparable KD as the parent antibody. Dissociation rate constants (koff values) ≤ 1 × 10−4 s−1 could not be reliably measured by SPR under the experimental conditions used
Table 2. Antigen-binding kinetics of anti-IL-17 antibody variants. Binding kinetics of the anti-IL-17 variants for IL-17FF were measured by SPR at 37°C, pH 7.4 and data fitted to a 1:1 binding model
Table 3. Antigen-binding kinetics and viscosity of anti-GCGR antibody variants. Binding kinetics of the anti-GCGR antibody variants for GCGR were measured by SPR at 37°C, pH 7.4 and data fitted to a 1:1 binding model. *Variants that reduced viscosity by >2-fold and maintained KD within 2-fold of the parent antibody. The viscosity was measured at 180 mg/mL final concentration in 20 mM histidine-acetate buffer at pH 5.5
