Figures & data
Figure 1. Lack of in vitro plasma stability translation to in vivo results. Correlation of mouse in vivo stability with in vitro stability for 13 TDC conjugates in frozen mouse plasma (a and b) and unfrozen mouse plasma (c and d) at 24 hr or 96 hr where 0 = no loss (stable) and 100 = complete loss (unstable); “loss” = linker-drug deconjugation and/or inactivating payload metabolism. Axes represent percent loss of drug relative to 0 h. (e, f) Specific examples of mouse plasma stability of MS spectra DAR profiles (top, middle) showing disconnect with in vivo stability (bottom) for an anti-HER2 TDC conjugated with an anthracycline analog (PNU) (e) and an anti-HER2 TDC conjugated to MMAE (f). MS peak labels indicate an antibody (Ab, glycated (Glc)) with one linker drug (+ LD) or two (+ 2*LD) can have drug lost and replaced by a cysteine (+ Cys) or a glutathione (+ GSH) or modified (-E)
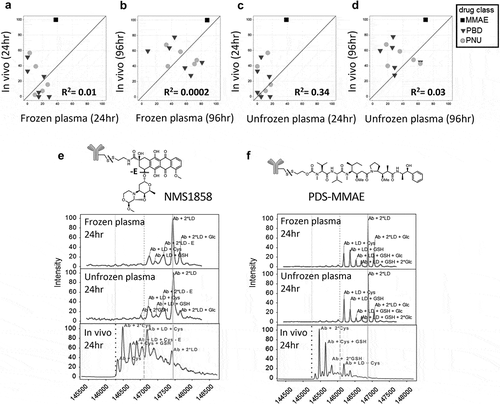
Figure 2. Improved correlation of in vitro stability with in vivo stability using whole blood. Correlation of TDC in vivo stability with unfrozen whole blood compared to in vivo at 24 h (a) using the same 13 conjugates evaluated in plasma (). (b, c) Specific examples of MS spectra of in vivo DAR stability profiles (B, C bottom) compared to stability profile in whole blood (top) for an anti-HER2 TDC conjugated with an anthracycline analog (PNU) (b) and an anti-HER2 TDC conjugated to MMAE (c). Axes in (A) represent the loss of drug percentage relative to 0 hr. MS peak labels indicate an antibody (Ab, glycated (Glc)) with one linker drug (+ LD) or two (+ 2*LD) can have drug lost and replaced by a cysteine (+ Cys) or a glutathione (+ GSH) or modified (-E). A 96 h time-point in whole blood was not evaluated because, after 48 h at 37°C, the samples could not be processed due to clotting of the whole blood
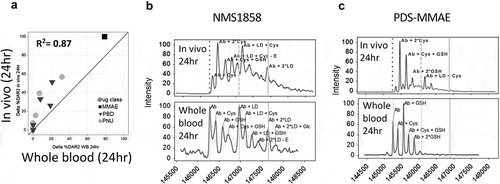
Figure 3. Identification of stability outliers between mouse in vitro whole blood and in vivo outcomes. Additional TDC conjugates were evaluated for stability and their in vitro and in vivo correlation compared (a). TDC conjugates outside of the ± 20% (dashed line) of linear regression line (solid line) were identified as outliers and spectra are shown (B, C, D). Axis in (A) represents a change in DAR2 at 24 h in matrix relative to buffer at 0 hr. MS peak labels indicate an antibody (Ab, glycated (Glc)) with one linker drug (+ LD) or two (+ 2*LD) can have drug lost and replaced by a cysteine (+ Cys) or a glutathione (+ GSH) or modified (acetyl loss – Ac, methyl loss – Me, amide and ester hydrolysis – B)
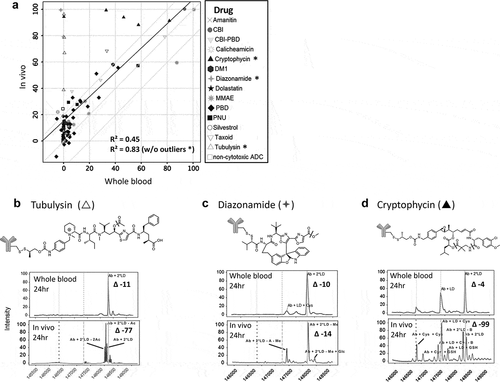
Figure 4. In vitro to in vivo stability translation in other species. Correlation of in vitro TDC stability with in vivo stability in (a) rat (top), (b) cyno, and (c) human (bottom). Evaluated 11 conjugates for rat, 5 pyrrolobenzodiazepine conjugates with different linkers for cyno and 1 conjugate for human. (b) Specific example of a cyno plasma stability disconnect with cyno whole blood and in vivo for a TDC with an anthracycline analog. MS peak labels indicate an antibody (Ab, glycated (Glc)) with one linker drug (+ LD) or two (+ 2*LD) can have drug lost and replaced by a cysteine (+ Cys) or a glutathione (+ GSH) or modified (ether cleavage – E)
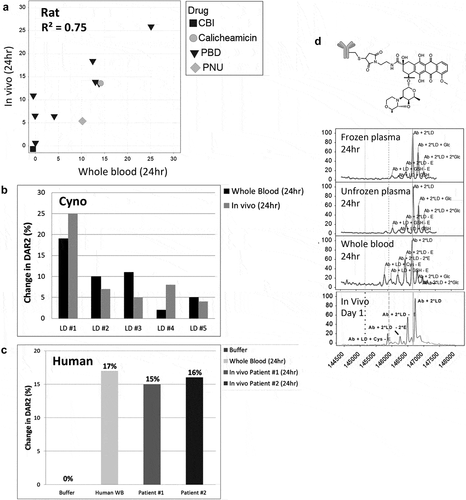
Figure 5. Reproducibility and species difference of whole blood stability for two control TDCs (a, b) using whole blood shipped overnight (n = 13). Differences in drug modification (b ether cleavage (-E), e carbamate hydrolysis (-CB)) and DAR (c disulfide cleavage, f maleimide cleavage) between buffer and four species for two different conjugates (b, c and e, f) run as controls for each shipment of whole blood
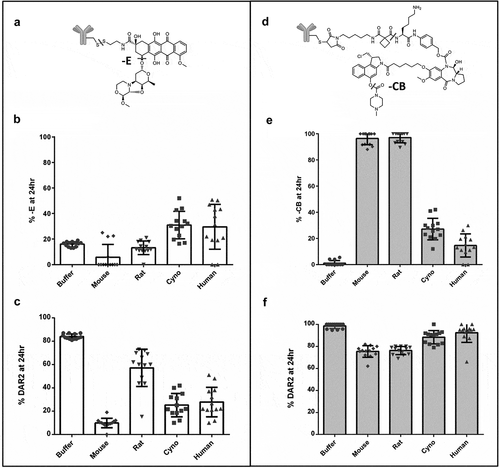
Table 1. Differences in DAR and drug modification between buffer and matrix from four species for two different conjugates run as controls with each shipment of whole blood
