Figures & data
Figure 1. Inhibition of PI3K signaling potently inhibits rotavirus infection. (A) Western blot analysis of p-mTOR (S2448), t-mTOR, p-Akt (S473), p-S6 (S240/244) and p-4E-BP1 (T70) protein levels and the corresponding total protein levels under the treatment of indicated concentrations of LY294002 (48 h) in Caco2 cells. (B) Treatment with LY294002 (48 h) potently inhibited viral genomic RNA in SA11 rotavirus infected Caco2 cells measured by qRT-PCR (n = 4, mean ± SEM, #P < 0.05, Mann-Whitney test). (C) Effects of LY294002 on the production of infectious viral particles determined by TCID50 method. Each bar represents the TCID50/mL (mean ± SEM) (n = 5, #P < 0.05, ##P < 0.01, Mann-Whitney test). (D) Treatment with LY294002 (48 h) potently inhibited viral VP4 protein in SA11 rotavirus infected Caco2 cells determined by western blot assay. (E) Representative confocal immunostainings of p-S6 (S240/244) (Green) after 48 h incubation with 0 (as control) and 5 μM LY294002 in human intestinal organoids. Nuclei were visualized by DAPI (blue). (F) Treatment with LY294002 (48 h) significantly inhibited viral genomic RNA in SA11 rotavirus infected human intestinal organoids examined by qRT-PCR (n = 4, mean ± SEM, #P < 0.05, Mann-Whitney test).
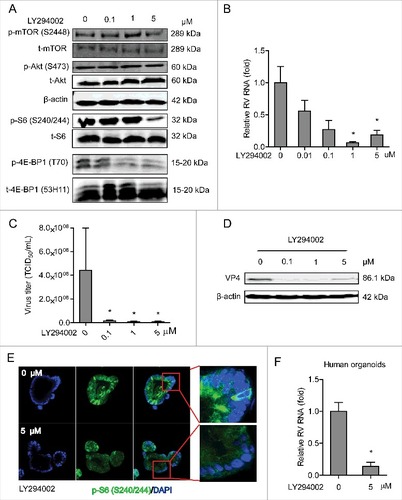
Figure 2. Silence of mTOR inhibits rotavirus replication. (A) Western blot analysis of t-mTOR, p-S6 (S240/244), t-S6, p-4E-BP1 (T70) and t-4E-BP1 (53H11) protein in lentiviral RNAi against mTOR transduced Caco2 cells. (B) One (No. 8198) of 3 lentiviral shRNA vectors showed successful knockdown determined by qRT-PCR (n = 9, mean ± SEM, ###P < 0.001, Mann-Whitney test). (C) mTOR knockdown inhibited rotavirus genomic RNA determined by qRT-PCR (n = 7, mean ± SEM, #P < 0.05, Mann-Whitney test).
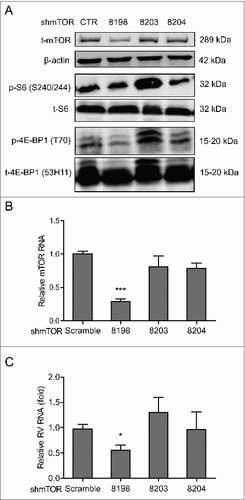
Figure 3. Rapamycin inhibits replication of SA11 and patient-derived rotavirus replication. (A) Western blot analysis of p-mTOR (S2448), t-mTOR, p-Akt (S473), t-Akt, p-S6 (S240/244), t-S6, p-4E-BP1 (T70) and t-4E-BP1 (53H11) protein levels with the treatment of indicated concentrations of rapamycin in Caco2 cells. (B) Treatment with rapamycin (48 h) significantly inhibited SA11 rotavirus replication in Caco2 cells (n = 7–9, mean ± SEM, ##P < 0.01, Mann-Whitney test). (C) Effects of rapamycin on the production of infectious viral particles determined by TCID50 method. Each bar represents the TCID50/mL (mean ± SEM) (n = 5, #P < 0.05, ##P < 0.01, Mann-Whitney test). (D) Treatment with rapamycin (48 h) inhibited viral VP4 protein determined by western blot assay in Caco2 cells. (E) Anti-rotavirus effect of rapamycin was abolished in mTOR knockdown Caco2 cells (n = 10, mean ± SEM, #P < 0.05, Mann-Whitney test). (F) Representative confocal immunostainings of p-S6 (S240/244) (Green) after 48 h treatment with 10 and 100 nM rapamycin in human intestinal organoids. Nuclei were visualized by DAPI (blue). (G) Treatment with rapamycin (48 h) inhibited SA11 rotavirus genomic RNA in human intestinal organoids determined by qRT-PCR (n = 5, mean ± SEM, #P < 0.01, Mann-Whitney test). (H) Treatment with 10 nM rapamycin (48 h) inhibited patient-derived rotavirus (isolate 1–5) genomic RNA in Caco2 cells determined by qRT-PCR. (I) Treatment with rapamycin (48 h) inhibited patient-derived rotavirus (isolate 1–5) genomic RNA in human intestinal organoids determined by qRT-PCR.
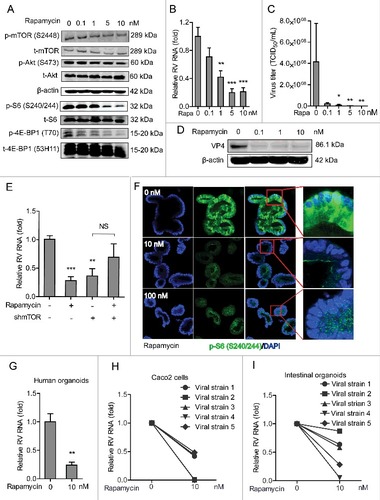
Figure 4. Dual inhibition of PI3K and mTOR inhibits rotavirus infection. (A) Western blot assay detected p-mTOR (S2448), p-p70S6K (T389), p-Akt (S473) and p-S6 (S240/244) and the corresponding total proteins after 48 h incubation with indicated concentrations of BEZ235 in Caco2 cells. (B) Treatment with BEZ235 (48 h) significantly inhibited SA11 rotavirus genomic RNA in dose-dependent-manner determined by qRT-PCR in Caco2 cells (n = 5, mean ± SEM, #P < 0.05, Mann-Whitney test). (C) Effects of BEZ235 on the production of infectious viral particles determined by TCID50 method. Each bar represents the TCID50/mL (mean ± SEM) (n = 5, #P < 0.05, ##P < 0.01, Mann-Whitney test). (D) Western blot showed that treatment with BEZ235 (48 h) significantly inhibited SA11 rotavirus VP4 protein in Caco2 cells. (E) Representative confocal immunostainings of p-S6 (S240/244) (Green) after 48 h incubation with 0 (as a control) and 10 nM BEZ235 in human intestinal organoids. Nuclei were visualized by DAPI (blue). (F) Treatment with BEZ235 (48 h) significantly inhibited SA11 rotavirus genomic RNA in human intestinal organoids quantified by qRT-PCR (n = 11, mean ± SEM, ##P < 0.01, Mann-Whitney test).
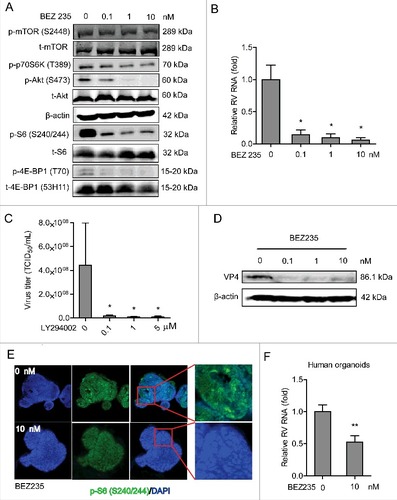
Figure 5. 4E-BP1 is a downstream effector of PI3K-Akt-mTOR signaling in sustaining rotavirus infection. (A) Western blot assay detected t-Akt and t-4E-BP1 (53H11) in lentiviral RNAi against 4E-BP1 transduced Caco2 cells. (B) Knockdown of 4E-BP1 significantly inhibited SA11 rotavirus genomic RNA quantified by qRT-PCR (n = 5, mean ± SEM, #P < 0.05, Mann-Whitney test). (C) Anti-rotavirus effect of rapamycin (10 nM) was abolished in 4E-BP1 knockdown Caco2 cells (n = 5, mean ± SEM, #P < 0.05, ##P < 0.01, Mann-Whitney test). (D) Western blot assay confirmed that anti-rotavirus effect of rapamycin (10 nM) was abolished in 4E-BP1 knockdown Caco2 cells. (E) Western blot indicated bona fide knockdown of 4E-BP1 in knockout (−/−) MEF cells. (F) SA11 rotavirus replication was potently restricted in 4E-BP1 knockout (−/−) MEF cells (n = 4, mean ± SEM, #P < 0.05, Mann-Whitney test). (G) Anti-rotavirus effect of rapamycin (10 nM) was attenuated in 4E-BP1 knockout (−/−) MEF cells (n = 8, mean ± SEM, #P < 0.05, Mann-Whitney test). (H) Rapamycin inhibited rotavirus replication in 4E-BP1 KO MEFs transfected with 4E-BP1-WT plasmids; while this antiviral effect was abolished in 4E-BP1 KO MEFs transfected with 4E-BP1–5A plasmids (n = 5, mean ± SEM, #P < 0.05, Mann-Whitney test).
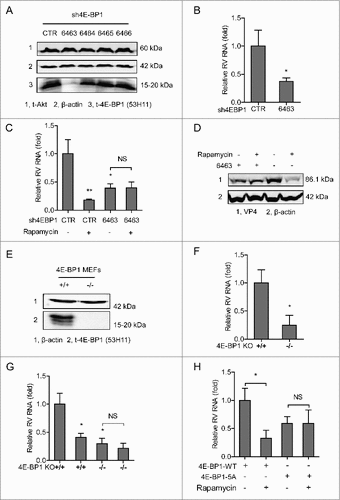
Figure 6. 4E-BP1 mediates rapamycin-induced autophagy that inhibits rotavirus infection. (A) Caco2 cells transduced with lentiviral particles carrying a construct of TagGFP2-LC3 driven by the elongation factor-1 promotor were cultured at 37°C for 48 h in DMEM medium containing EBSS medium containing 1mM pepstatin A and E-64-d solution (for starvation) and 10 nM rapamycin. LC3-positive puncta was observed by confocal laser microscopy. (B) LC3-I and LC3-II protein levels were examined by western blot analysis. Protein samples were extracted from Caco2 cells treated with indicated concentrations of rapamycin (48 h). Quantification of the intensity of the immunoreactive bands of both LC3-I and LC3-II was performed using Odyssey V3.0 software. Densitometric analysis of immunoblots of LC3 was expressed as the ratio of LC3-II to LC3-I, and the ratio of LC3II/LC3I was expressed in arbitrary units. (C) Western blot visualized LC3-I and LC3-II protein levels in starvation (EBSS medium containing 1mM pepstatin A and E-64-d solution) treated Caco2 cells. The ratio of LC3II/LC3I was expressed in arbitrary units. (D) Starvation significantly inhibited rotavirus RNA in Caco2 cells (n = 6, mean ± SEM, #P < 0.05, Mann-Whitney test). (E) Starvation dramatically inhibited rotavirus protein VP4 synthesis in Caco2 cells. (F) Rapamycin induced autophagy in 4E-BP1 KO MEFs transfected with 4E-BP1-WT plasmids; while the induction was abolished in 4E-BP1 KO MEFs transfected with 4E-BP1–5A plasmids. (G) Western blot demonstrated that upregulated ratio of LC3-II to LC3-I by rapamycin was abolished in 4E-BP1 knockout (−/−) MEFs (n = 6, mean ± SEM, #P < 0.05, ##P < 0.01, Mann-Whitney test). (H) Western blot detected that upregulated ratio of LC3-II to LC3-I by starvation (EBSS medium containing 1mM pepstatin A and E-64-d solution) was abolished in 4E-BP1 knockout (−/−) MEFs (n = 5, mean ± SEM, #P < 0.05, Mann-Whitney test).
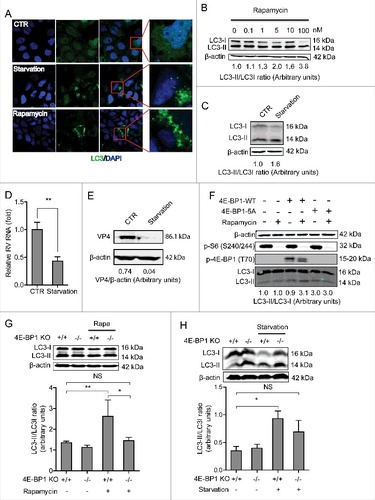
Figure 7. Silence of autophagy related genes, LC3-II and beclin1, inhibits rotavirus replication. (A) Western blot detected LC3-II protein in lentiviral RNAi against LC3-II transduced Caco2 cells. (B) LC3-II knockdown increased rotavirus genomic RNA determined by qRT-PCR (n = 4–10, mean ± SEM, #P < 0.05, ##P < 0.01, Mann-Whitney test). (C) LC3-II knockdown increased rotavirus VP4 protein synthesis detected by western blot assay. (D) Western blot detected beclin1 protein in lentiviral RNAi against beclin1 transduced Caco2 cells. (E) Beclin1 knockdown increased rotavirus genomic RNA determined by qRT-PCR (n = 11–17, mean ± SEM, ###P < 0.001, Mann-Whitney test). (F) Beclin1 knockdown increased rotavirus VP4 protein synthesis detected by western blot assay.
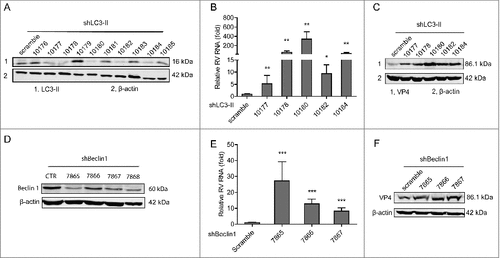
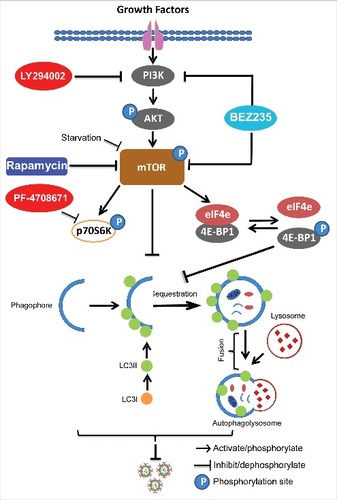
Figure 8. Schematic depicting the PI3K-Akt-mTOR signaling involved in regulating rotavirus replication. Pharmaceutical blocking PI3K-Akt-mTOR pathway inhibits phosphorylation and activation of its downstream targets including p70S6K and 4E-BP1. 4E-BP1 mediates inhibition of mTOR on autophagy machinery via 4E-BP1. Autophagy itself exerts anti-rotavirus effect.