Figures & data
Figure 1. Identification of C. tropicalis PKA catalytic subunits (Tpk1 and Tpk2). (A) The amino acid identity of Tpk proteins from C. albicans (Ca), C. tropicalis (Ct), and S. cerevisiae (Sc). Numbers above the diagonal line indicate sequence identity between the full-length proteins, while numbers below represent the identity of PKA domain (accesion:cd05580). (B) Phylogenetic tree of the PKA catalytic subunits of C. albicans, C. tropicalis, and S. cerevisiae. The amino acid identity and the multiple alignment were analyzed and constructed using the Clustal Omega program (http://www.ebi.ac.uk/Tools/msa/clustalo/). The phylogenetic tree was constructed in MEGA7 [Citation68] software using Maximum Likelihood method based on the JTT matrix-based model [Citation69]. The black bar indicates an evolutionary distance of 0.05 substitutions per site. Proteins and GenBank accession numbers: CaTpk1, XP_723574.1; CaTpk2, XP_714866.2; CtTpk1, XP_002549018.1; CtTpk2, XP_002547429.1; ScTpk1, NP_012371.2; ScTpk2, NP_015121.1; ScTpk3, NP_012755.1.
![Figure 1. Identification of C. tropicalis PKA catalytic subunits (Tpk1 and Tpk2). (A) The amino acid identity of Tpk proteins from C. albicans (Ca), C. tropicalis (Ct), and S. cerevisiae (Sc). Numbers above the diagonal line indicate sequence identity between the full-length proteins, while numbers below represent the identity of PKA domain (accesion:cd05580). (B) Phylogenetic tree of the PKA catalytic subunits of C. albicans, C. tropicalis, and S. cerevisiae. The amino acid identity and the multiple alignment were analyzed and constructed using the Clustal Omega program (http://www.ebi.ac.uk/Tools/msa/clustalo/). The phylogenetic tree was constructed in MEGA7 [Citation68] software using Maximum Likelihood method based on the JTT matrix-based model [Citation69]. The black bar indicates an evolutionary distance of 0.05 substitutions per site. Proteins and GenBank accession numbers: CaTpk1, XP_723574.1; CaTpk2, XP_714866.2; CtTpk1, XP_002549018.1; CtTpk2, XP_002547429.1; ScTpk1, NP_012371.2; ScTpk2, NP_015121.1; ScTpk3, NP_012755.1.](/cms/asset/da4da8da-3437-4f6b-8d53-053b2af3a697/kvir_a_1414132_f0001_b.gif)
Figure 2. Protein kinase A is required for growth. (A) Growth curves of C. tropicalis wild type and mutants. Cells were grown overnight in YPD at 30°C, washed twice with dH2O, diluted to 0.01 OD600, except for the tpk1/tpk1 tpk2/tpk2 mutant (YEN1), which was diluted to 0.1 OD600 with fresh YPD medium, and incubated at 30°C and 200 rpm for four days. The OD600 of strains was measured via microplate spectrophotometer at the indicated time. The experiments were performed in triplicate, and the values represent the mean ± the standard error of the mean. (B) Spot assay of the indicated strains at different temperatures. Cells were grown overnight in YPD at 30°C (except the tpk1/tpk1 tpk2/tpk2 mutant, which was grown for two days), washed twice with dH2O, and diluted to 0.2 OD600 as the starting concentration. Cells were then five-fold serially diluted, spotted onto YPD medium, and incubated at 25°C, 30°C, 37°C, or 42°C for 48 h and photographed. (C) Protein kinase A activity of the indicated strains. Cells were grown overnight in YPD at 30°C (except the tpk1/tpk1 tpk2/tpk2 mutant, which was grown for two days) and washed twice with dH2O. Crude protein extracts of the samples were isolated. PKA activity assays were carried out using the PepTag assay for non-radioactive detection of cAMP-dependent protein kinase kit.
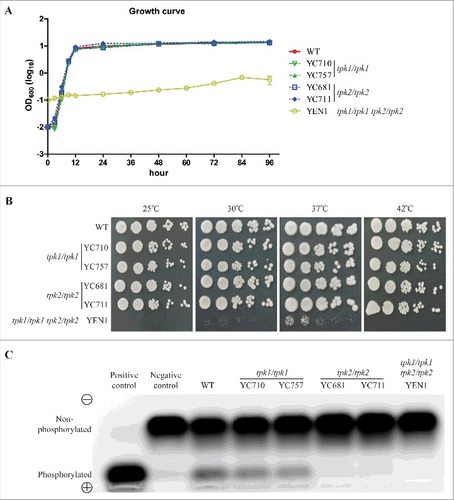
Table 1. C. tropicalis strains used in this study.
Figure 3. Tpk1 contributes to cell wall integrity and drug tolerance. Growth of the indicated strains exposed to cell wall-perturbing agents, antifungal drugs, and various stresses. Cells were grown overnight in YPD at 30°C (except tpk1/tpk1 tpk2/tpk2 mutant, which was grown for two days), washed twice with dH2O, and diluted to 0.2 OD600 as the initial concentration. Samples were then five-fold serially diluted, spotted onto YNB medium containing the indicated chemicals, and incubated at 30°C for 48 h. MCF, micafungin; ANF, anidulafungin; PSC, posaconazole; SDS, sodium dodecyl sulfate; DTT, dithiothreitol.
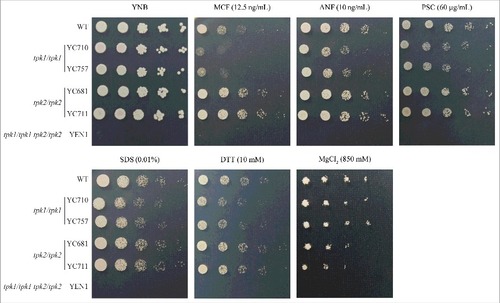
Figure 4. Tpk2, but not Tpk1, regulates hyphal growth. (A) Hyphal growth of the indicated strains in liquid media. Cells were grown overnight in YPD at 30°C (except tpk1/tpk1 tpk2/tpk2 mutant, which was grown for two days), washed twice with dH2O, diluted to 0.2 OD600 with fresh SC medium or SC medium supplemented with 10 mM of GlcNAC or 3AT, and incubated at 37°C and 200 rpm. (B) Hyphal growth of the indicated strains on solid media. Cells were grown overnight in YPD at 30°C (except tpk1/tpk1 tpk2/tpk2 mutant, which was grown for two days), washed twice with dH2O, and diluted to 103 cells/mL. Then, 50 μL containing ∼50 cells were spread onto filament-inducing SLAD or Spider plates and incubated at 37°C for the indicated time.
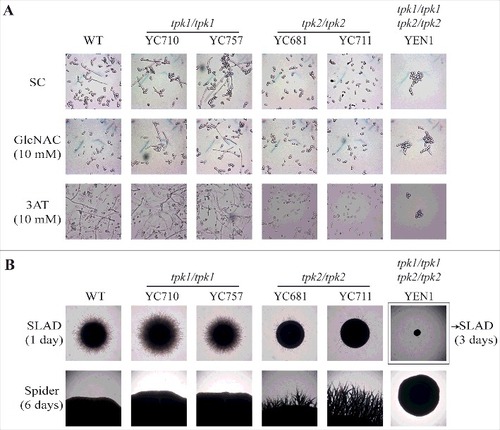
Figure 5. Tpk2 is involved in flocculation and biofilm formation. (A) Tpk2 is required for flocculation. Cells were grown overnight in SC medium containing 10 mM GlcNAc at 30°C (except the tpk1/tpk1 tpk2/tpk2 mutant, which was grown 2 days in YPD, washed twice with dH2O, transferred to SC medium containing 10 mM GlcNAc and incubated at 30°C overnight). The cells were then vortexed, centrifuged at 500 rpm for 1 min, and photographed. (B) Tpk2 is critical for biofilm formation. Cells were grown in YPD medium overnight at 30°C (except tpk1/tpk1 tpk2/tpk2 mutant, which was grown for two days), washed twice with dH2O, and diluted to 0.5 OD600 in SC medium. Then, 2 mL of each sample were inoculated into a 12-well plate for 90 min at 37°C and 200 rpm. The wells were then washed twice with PBS buffer, inoculated with 2 mL of fresh SC medium, and incubated for 24 h at 37°C and 200 rpm. The wells were then washed twice with PBS, stained with 0.05% crystal violet, and photographed.
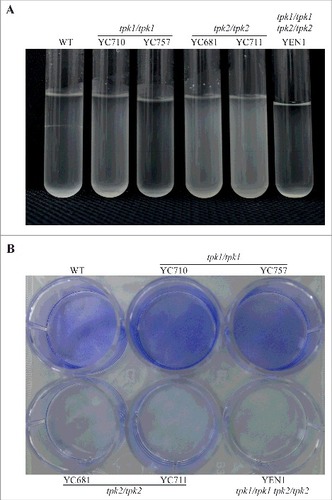
Figure 6. Both Tpk1 and Tpk2 are both required for full virulence. (A) Survival curves of mice infected with the indicated C. tropicalis strains. Cells were grown overnight in YPD medium (except tpk1/tpk1 tpk2/tpk2 mutant, which was grown for two days), washed three times with PBS buffer (pH 7.4), resuspended in PBS buffer, and diluted to a concentration equal to 2.5 × 107 CFU/mL. Mice were inoculated with with 5 × 106 C. tropicalis cells in 200 µL and were monitored for 33 days. Ten mice per strain were used, except for the wild-type strain for which nine were used because one mouse died during the infection process. (B) The fungal burden in the kidneys and brains was measured on day 3 post-C. tropicalis infection in five mice per strain.
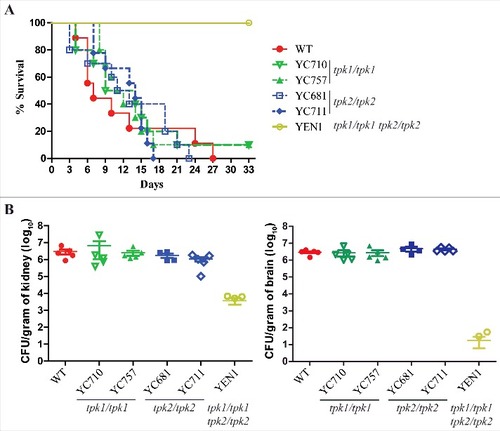
Figure 7. Both Tpk1 and Tpk2 are required for colonization and necrosis in kidneys and brains. Histopathological analyses of mouse kidney and brain organs obtained from C. tropicalis-infected mice at day 3. Organs were fixed in 10% formaldehyde solution, paraffin-embedded, and stained with PAS and H&E to observe C. tropicalis colonization and tissue necrosis, respectively. White arrowheads indicate fungal cells (hyphae or yeasts). Scale bar = 50 µm.
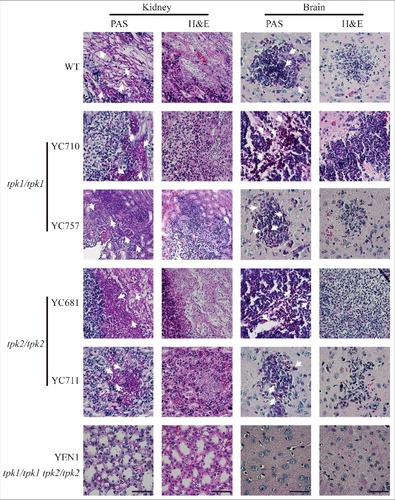
Figure 8. Tpk1 and Tpk2 are involved in the regulation of virulence-related gene expression. Cells were grown overnight in YPD at 30°C, washed twice with dH2O, diluted to 0.1 OD600 in 10 mL of Spider medium, and incubated for 4 h at 37°C. Total RNA was extracted using TRIzol and treated with the TURBO DNA-free™ Kit to remove genomic DNA. cDNA synthesis was carried out using 200 ng of RNA with the high-capacity cDNA reverse transcription kit. Transcript expression levels were analyzed by SYBR® Green PCR Master Mix and normalized to the C. tropicalis ACT1 gene using method. Asterisks indicate statistically significant differences compared with the wild type using Dunnett's multiple comparison test compared to wild type (#P < 0.05; ##P < 0.01; ###P < 0.001).
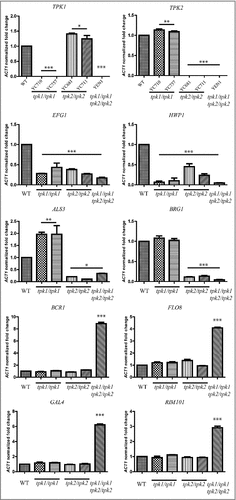
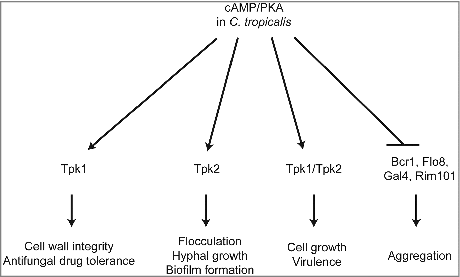
Figure 9. Proposed roles of PKA in C. tropicalis. The C. tropicalis PKA catalytic subunit Tpk1 controls cell wall integrity and drug tolerance, while Tpk2 is required for flocculation, hyphal growth, and biofilm formation. In addition, both proteins are not essential in C. tropicalis and have redundant roles in growth and virulence; loss of either gene results in growth and virulence similar to the wild type. In contrast, Tpk1 and Tpk2 negatively regulate expression of adherence-associated transcription factors (Bcr1, Flo8, Gal4, and Rim101), which were upregulated in the tpk1/tpk1 tpk2/tpk2 mutant that showed cell aggregation.