Figures & data
Figure 1. Differential S. suis 2 two-component signal transduction system response regulator genes expression within the PMN environment. PMNs were incubated with SC19 for 180 min, after which bacterial RNA was isolated and purified, and expression levels were measured by qRT-PCR. The relative expression level of each gene was normalized to that of the housekeeping genes proS and 16S rRNA. ##p < 0.01 and ###p < 0.001 compared to untreated SC19.
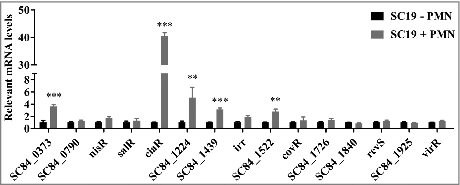
Figure 2. Characterization of mutant strain ΔvraSR. (A) Transmission electron micrographs of S. suis. Scale bar: 200 nm. (B) The thickness of the bacterial capsules. Data are the result of 36 bacterial capsules analyzed per sample using Image J software. (C) Concentrations of sialic acid in the S. suis strains. (D) Expression of capsule synthesis-associated genes in vitro. (E) Growth curves for the strains grown in TSB with 10% serum. Bacterial cell density was measured with a spectrophotometer at 600 nm. #p < 0.05, ##p < 0.01 and ###p < 0.001 compared to vraSR mutant strain.
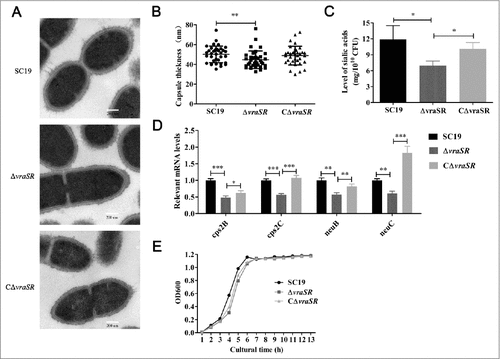
Figure 3. Kinetics of S. suis killing or phagocytosis by human blood, PMNs, and bactericidal substances. (A) Absence of vraSR significantly attenuates the ability of S. suis 2 to survive in human blood. (B) Decreasing survival ability of ΔvraSR over time in the PMN environment. At each time point, PMNs were lysed and viable bacteria were counted on TSA. Results of representative experiments are shown. (C) Phagocytosis of S. suis strains by human PMNs. SC19, ΔvraSR and CΔvraSR were labeled with FITC and incubated with PMNs for different lengths of time. Flow cytometry was used to measure the phagocytosis efficiency. (D) Killing of SC19, ΔvraSR and CΔvraSR S. suis strains by H2O2. (E) Killing of SC19, ΔvraSR and CΔvraSR S. suis strains by lysozyme. S. suis cultures in exponential phase were incubated with gradient concentrations of H2O2 or lysozyme at 37 °C for 30 min, and the numbers of viable cells were determined. #p < 0.05, ##p < 0.01 and ###p < 0.001 compared to vraSR mutant strain.
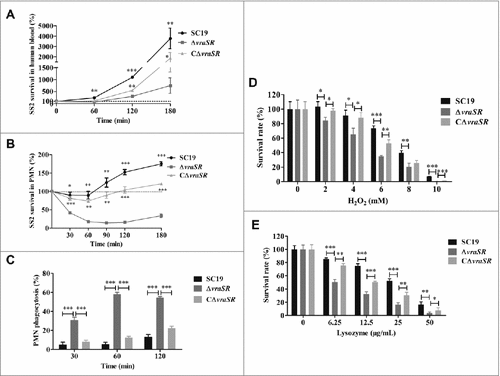
Figure 4. HBMEC cell adhesion assays. (A) Ability of S. suis strains to adhere to HBMEC cells. (B) Adhesion-associated gene expression of different strains in vitro. Total RNA was extracted from SC19, ΔvraSR and CΔvraSR grown in TSB medium with 10% serum at mid-log phase and used for qRT-PCR. Results are shown as the relative expression ratio compared with their expression in the parental strain SC19. These data represents the means of triplicate values. #p < 0.05, ##p < 0.01 and ###p < 0.001 compared to vraSR mutant strain.
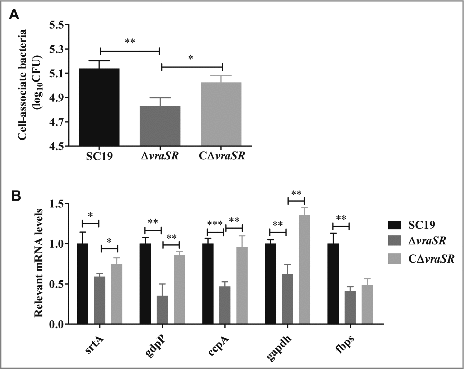
Figure 5. Virulence of ΔvraSR in a BALB/c mouse model. (A) Survival curves for mice infected with S. suis strains. Differences in the survival rates between SC19 and the mutant strains were analyzed with the log-rank test (##p < 0.01). (B) Bacterial burdens in the blood (CFU/mL of blood), and in the heart, liver, spleen, lung, kidney and brain (CFU/g of tissue). ##p < 0.01 and ###p < 0.001 compared to vraSR mutant strain.
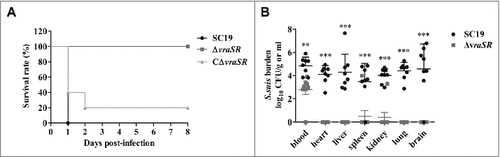
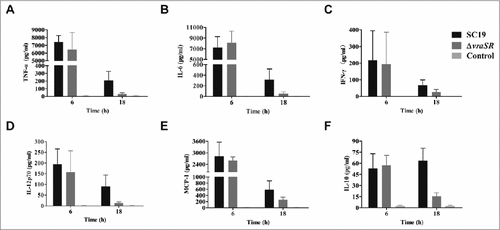
Figure 6. Production of inflammatory cytokines in vivo during pathogenic S. suis 2 infection. BALB/c mice were injected intraperitoneally with 5 × 107 CFU of SC19 or ΔvraSR for 6 h or 18 h. Serum was harvested at each time point post-infection and the concentrations of cytokines were measured by a CBA Mouse Inflammation Kit. (A–F) Concentrations of TNF-α (A), IL-6 (B), IFN-γ (C), IL12p70 (D), MCP-1 (E), and IL-10 (F) in the serum. Results are displayed from three infected mice per group at each indicated time.