Figures & data
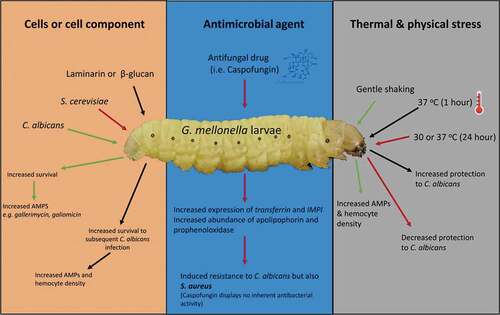
Figure 1. Comparision between mammalian and invertebrate Toll/Toll-like receptor and IMD/TNF-α signaling. Upon activation of invertebrate Toll receptor and the homologous Toll-like receptor in vertebrates, a cascade is induced where the homologous transcription factors Nf-κB and Dif are activated in vertebrates and invertebrates, respectively. The IMD pathway is activated by binding of peptidoglycan (PGN) to peptidoglycan-recognition proteins (PGRPs) which results in recruitment and formation of an IMD, dFADD and DREDD complex and results in IMD cleavage and activation of TAB2/TAK1. This results in Relish phosphorylation and ultimately the production of AMPs (e.g. cecropin). In mammals, TNF-α is bound by the tumor necrosis factor receptor 1 (TNF-R1) which results in recruitment of RIPP, FADD, and caspase 8. NF-κB is released from its inhibitor protein (IκB) via phosphorylation by IKK complex which results in NF-κB translocation to the nucleus resulting in pro-inflammatory cytokine production
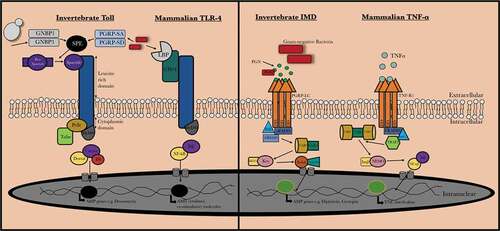
Figure 2. Summary of immune priming in G. mellonella larvae. The effect of cells or cell component, antimicrobial agents or thermal and physical stress on immune priming in G. mellonella larvae. Components of the fungal cell wall (laminarin/β-glucan) or a sub-lethal C. albicans or S. cerevisiae infection protect from a subsequent potentially lethal infection by an increased abundance of AMPs and the number of circulating hemocytes. The antifungal agent caspofungin induced increased resistance to S. aureus infection. Gentle shaking and a 1 h 37°C incubation induces immune priming in larvae