Figures & data
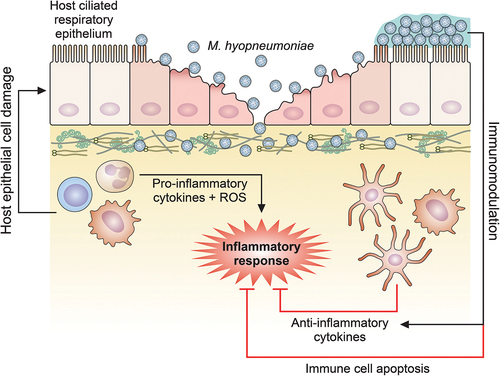
Figure 1. Schematic representation of M. hyopneumoniae adhesion to swine ciliated respiratory epithelium. (a) M. hyopneumoniae cells attach to the cilia of respiratory epithelium, causing ciliostasis, cilium loss, and subsequent epithelial cell death. M. hyopneumoniae cells may form biofilms on the ciliated epithelial surface. M. hyopneumoniae also interacts with molecules from the ECM, such as fibronectin and plasminogen. (b) M. hyopneumoniae adhesion to ciliated cells is mediated by bacterial adhesins that interact with host ligands, as GAGs (displayed on cilia surface), and extracellular actin. (c) Adhesins of M. hyopneumoniae are endoproteolytically processed by surface-displayed proteases, generating a combinatorial library of adhesin proteoforms exposed on the bacterial surface. Dashed lines represent damaged ciliated epithelial cells
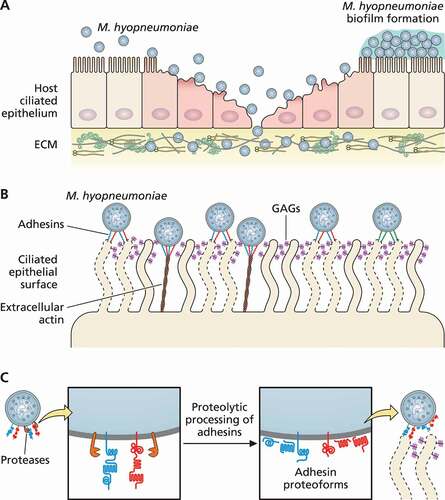
Table 1. M. hyopneumoniae surface adhesins and adhesion-related proteins and their host ligands
Table 2. Putative virulence factors found in the M. hyopneumoniae secretome and/or surfaceome
Figure 2. Schematic representation of host immune response modulation mediated by M. hyopneumoniae. M. hyopneumoniae infection elicits an acute inflammatory response in swine lungs, represented by a prominent infiltration and accumulation of immune cells that secrete pro-inflammatory cytokines and release ROS, triggering a microbicidal response. However, this pro-inflammatory microbicidal response causes damage to the host respiratory epithelium. Moreover, M. hyopneumoniae cells can persist in the respiratory tract modulating the host immune response, eliciting the secretion of anti-inflammatory cytokines by dendritic cells and macrophages, and inducing apoptosis on immune cells accumulated in the respiratory tract. The representation of M. hyopneumoniae cells attached to the ciliated respiratory epithelium is described in Figure 1
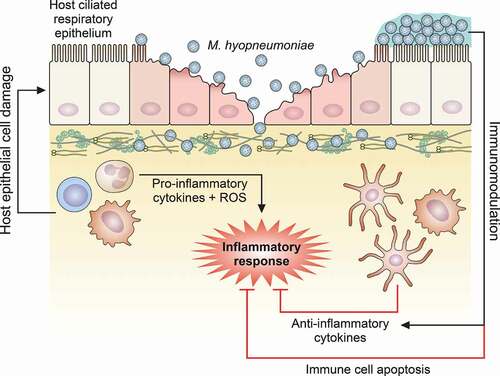
Figure 3. Schematic representation of host cell damage pathways induced by M. hyopneumoniae. M. hyopneumoniae cells interact with host ciliated epithelium causing cell damage through surface-displayed cytotoxic proteins and ROS release. These cytotoxic molecules can induce host cell death by triggering different pathways, including the apoptosis cascades associated with cytochrome C, caspase-3 and −8 (apoptosome), UPR, MAPK and NF-κB. Internalization of M. hyopneumoniae into a vesicle-like structure in the host cell cytosol and trafficking between the intra- and extracellular milieus are also represented. The representation of M. hyopneumoniae cells attached to the ciliated respiratory epithelium is described in Figure 1. ER, endoplasmic reticulum
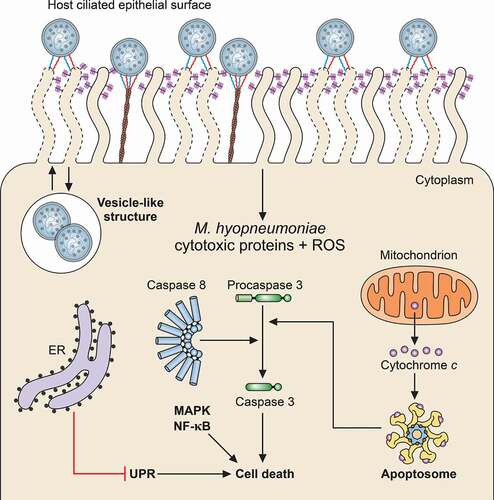
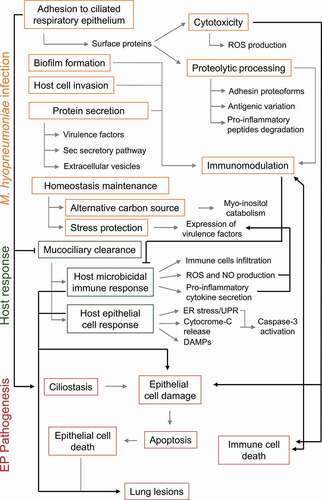
Figure 4. Schematic representation of M. hyopneumoniae pathogenicity determinants and host responses involved in EP establishment. Infection success depends on M. hyopneumoniae capacity to escape from the host mucociliary clearance and to adhere to ciliated cells of the porcine respiratory epithelium. Upon adhesion, M. hyopneumoniae causes ciliostasis and may form biofilms or invade host epithelial cells. M. hyopneumoniae also has a cytotoxic effect on host cells, causing cell damage and epithelial and immune cell death by apoptosis. This activity contributes to the modulation of the induced host immunological responses and may lead to lung lesions. Cell damage and epithelial cell death is also an outcome of host microbicidal and epithelial response to M. hyopneumoniae infection. Immunomodulation may also be associated with bacterial antigenic variation generated by proteolytic processing of surface adhesins and other surface proteins, and with the secretion of several virulence factors, as well as host pro-inflammatory peptides. During infection, M. hyopneumoniae may maintain its homeostasis using myo-inositol from lung tissue as an alternative carbon source. M. hyopneumoniae must also deal with stressing conditions, and its exposure to the host environment triggers the expression of several virulence factors. M. hyopneumoniae pathogenicity mechanisms, host response mechanisms and EP pathogenesis outcomes are shown in orange, green and red rectangles, respectively. Molecular processes and molecules involved in each mechanism or outcome are pointed out by gray arrows. Associations among M. hyopneumoniae pathogenicity mechanisms, host responses and EP pathogenesis outcomes are pointed out by black arrows
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
