Figures & data
Figure 1. Structure and phosphorylation of HPK. The HPK is a dimer composed of two subunits. Each subunit contains an ATP binding domain, a dimerization domain, and a kinase domain (phosphorylation site). When the input domain of HPK is appropriately stimulated, the dimerization domain of one subunit will approach to the kinase domain of the other subunit to promote the phosphorylation
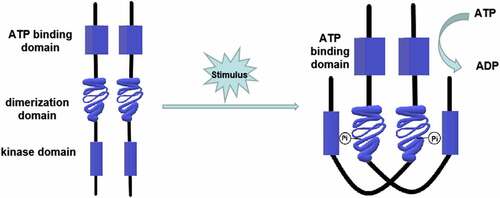
Figure 2. One-step phosphorylation of His-Asp in prokaryotes. A HPK is autophosphorylated on a histidine residue and the signal is subsequently transferred to a RR on an aspartate residue. The phosphorylated RR acts as a transcription factor regulating gene expression or a protein activity regulator. The transfer of phosphate acid from HPK to RR takes only one step (His-Asp)
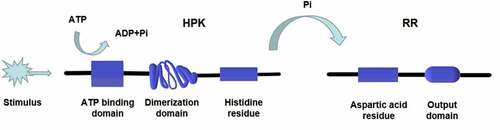
Figure 3. The two-component systems and the downstream pathways in different fungi. The two-component system in most eukaryotes is a multistep phosphate transduction model. The structure and conduction pathway of the two-component system are different in various fungi. For example, S. cerevisiae expresses only one HPK, C. albicans contains 3 HPKs, and C. neoformans has 7 HPKs. The phosphorylation level of HPK affects the phosphorylation rate of RR. Multiple HPKs may regulate one RR, while one HPK may also regulate multiple RRs
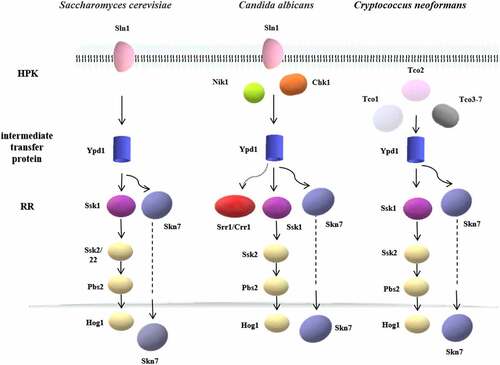
Figure 4. Multistep phosphorylation of His-Asp in fungi. After the HPK detects the stimulus signal, ATP is used as the donor to phosphorylate a conserved his residue. Subsequently, the phosphate group is transferred to the Asp residue of the same HPK receptor domain and then transferred to the Asp residue of the RR receptor domain through the his residue of intermediate transfer protein. Four phosphorylation events occur in sequence, forming the four-step phosphate transfer (His-Asp-His-Asp)
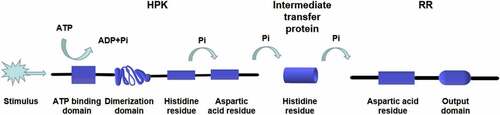
Figure 5. Two-component system of C. albicans and its downstream pathways. Seven proteins of the two-component system in C. albicans are shown, including three hybrid HPKs (Sln1p, Nik1p/Cos1p, Chk1p), three RRs (Ssk1p, Skn7p, Crr1p/Srr1p), and one intermediate transfer protein (Ypd1p). The downstream responses of two-component system are complex and diverse, which is highly related to morphogenesis, oxidative and osmotic stress, quorum sensing, virulence regulation and so on
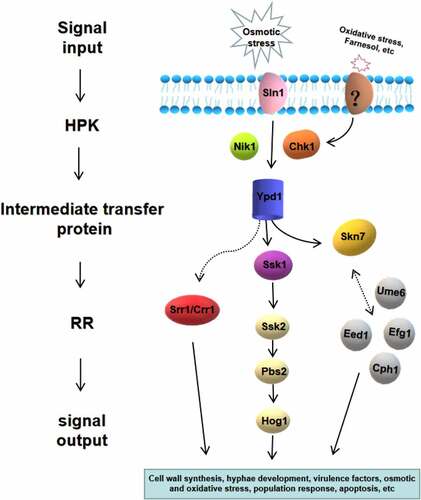
Table 1. Components and functions of the two-component system of C. albicans.
Figure 6. Regulation of C. albicans hyphal development by two-component system. Sln1p, Nik1p and Chk1p transfer the regulation signals to RR through Ypd1p. It is still unknown how to distinguish and transmit signals to the downstream RR (Ssk1p and Skn7p). The hyphal forms of NIK1 mutant cultured in 30°C liquid media is similar with the wild-type strain, while it was defective on a solid agar at 37°C. CHK1 mutants and SSK1 mutants have a hyphal formation defect on solid medium, but they can develop hyphae and flocculate extensively in liquid media. The deletion of YPD1 increased the hyphae formation and flocculation in liquid media. Overexpressing SKN7 in EED1, EFG1, CPH1 and UME6 mutants did not show the similar wrinkled and contained filamentous cells compared to that in wild type strain, suggesting that EED1, CPH1, UME6 and EFG1 are essential for SKN7 function in morphogenesis, but the mechanisms are still unknown
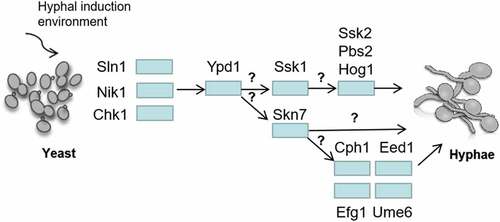
Figure 7. Regulation of C. albicans virulence by two-component system. The virulence of C. albicans with SLN1 or NIK1 deletion is decreased. CHK1 and SSK1 mutants are both nontoxic in the disseminated murine model of candidiasis, suggesting that HPKs and SSK1 positively regulate the virulence of C. albicans. The inhibition of the expression of YPD1 increased the virulence of C. albicans, indicating Intermediate transfer protein negatively regulated the virulence. SKN7 had little effects on the virulence of C. albicans, while the regulation of SRR1 on virulence is unclear. In the downstream MAPK pathway, both PBS2 and HOG1 mutants attenuated virulence in a mouse model, indicating that PBS2 and HOG1 positively regulate the virulence of C. albicans, while the regulation of SSK2 on virulence is still unknown
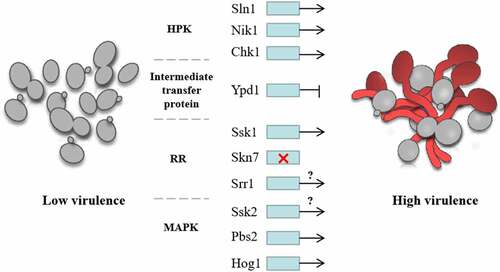
Figure 8. Regulation of osmotic stress response by two-component system. Sln1p acts as an osmotic sensor protein to regulate the Hog1-MAPK signal transduction system in C. albicans. When the cells are in an isoosmotic or hypoosmotic environment, phosphorylation of Ssk1p inhibits activation of the Hog1-MAPK cascade, but in hyperosmotic cells, unphosphorylated Ssk1p activates the Ssk2/22 MAPKKK and subsequent phosphorylation of Pbs2p and Hog1p. Finally, the phosphorylated Hog1p is transferred to the nucleus, which activates transcription factors to induce the expression of GPD1, increasing the intracellular glycerol content to adapt to hyperosmotic stress
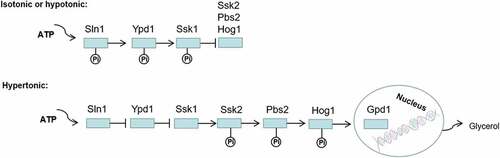
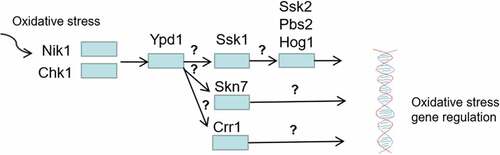
Figure 9. Regulation of oxidative stress response by two-component system. Among the three HPKs Nik1 and Chk1 are required for activation of Ypd1 in response to oxidative stress, then the three RRs (Ssk1, Skn7, Crr1) are activated to regulate oxidative stress by transmitting oxidative stimulation signals to different downstream proteins
Data availability statement
Data sharing does not apply to this article as no new data were created or analyzed in this study.
