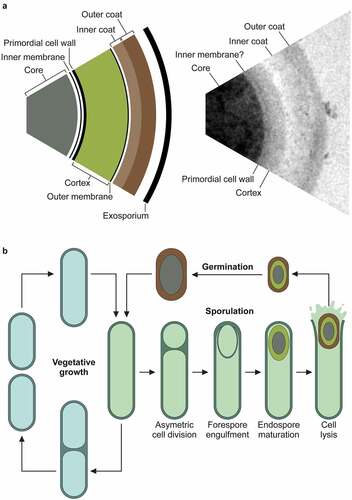
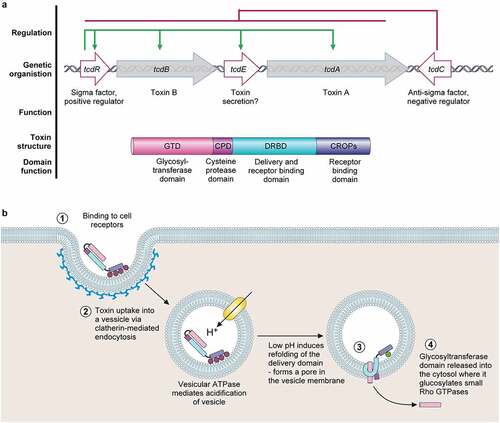
a. The pathogenicity locus (PaLoc) is composed of 5 genes:
tcdA and
tcdB, encoding toxins A and B respectively;
tcdR, encoding an alternative sigma factor and likely positive regulator of the PaLoc (regulation shown above in green);
tcdE, encoding a holin-like protein putatively involved in toxin secretion; and
tcdC, an anti-sigma factor and negative regulator of the PaLoc genes (regulation shown above in red). Toxins A and B both consist of a broadly similar four-domain structure. At the N-terminal, the glucosyltransferase domain (GTD) is the active toxin moiety which inactivates members of the Rho GTPase family. A cysteine protease domain is next to the GTD, and is involved in auto-processing and release of the GTD. The next domain, often called the Delivery and Receptor Binding Domain (DRBD), contains a hydrophobic region and is thought to be involved in translocation of the GTD from the lumen of endocytic vesicles into the host cell cytoplasm. The final C-terminal receptor-binding domain (also known as C-terminal combined repetitive oligopeptides (CROPS) domain) binds to a range of cellular receptors.
b. Toxin mode of action [
Citation65]. The toxins bind to various cellular receptors via the C-terminal CROPs domain, triggering clathrin-dependent endocytosis (
1) followed by acidification of the resulting vesicle (
2). The drop in pH triggers a conformational change in the delivery domain which inserts into, and forms a pore in, the vesicle membrane, through which the GTD transits into the host cytoplasm (
3). The GTD is then released by a cleavage event mediated by the cysteine protease domain, in a process that is dependent on host inositol hexakisphosphate (
4). The GTD is then able to glucosylate and inactive members of the small Rho GTPase family, including Rho, Rac, and Cdc42. Inactivation of Rho GTPases results in multi-level cellular disruption, including dysregulation of actin depolymerization, which causes disruption of tight junctions and loss of intestinal barrier function, induction of proinflammatory cytokines and activation of programmed cell death.