Figures & data
Figure 1. GAS persistence and proliferation occurs due to reduced phagocytosis and internalisation. (a) GFP GAS association with human neutrophils after 30 min incubation (n = 6 donors, Student’s t-test) via flow cytometry (Figure S1). (b) The internalisation of GAS by neutrophils determined by gentamicin protection assay (n = 5 donors, Student’s t-test). (c) Rate of ROS production by neutrophils between 30 and 60 min (n = 3 donors). GAS strains were incubated in the presence of (d) active human neutrophils (n = 8 donors) and (E) lysed neutrophils (n = 4 donors) over 180 min and percent survival determined. (F) GAS killing by human neutrophils at 30 min following pre-incubation with cytochalasin D to inhibit phagocytosis (n = 4 donors). (c-f) Holm-Šídák multiple comparison. Results are the pooled means ± SD of triplicate measurements. *p < 0.05, **p < 0.01 and ****p < 0.0001.
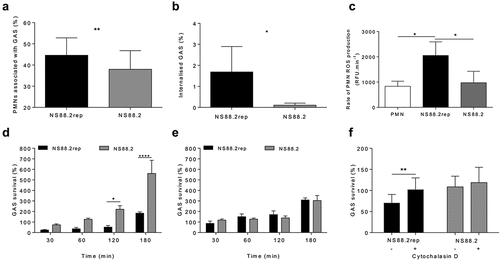
Figure 2. GAS infection induces neutrophil death. Human neutrophils (PMNs) were incubated with NS88.2, NS88.2rep or in the absence of GAS and stained with annexin V-FITC (AV) and Zombie Aqua viability dye (Z) before being analysed by flow cytometry. Neutrophils were gated as (a) singlets and then total (b) neutrophils (live and dead). Cells were analysed by fluorescence of annexin V-FITC and Zombie Aqua binding, where flow cytometry plots are representative and show (c) uninfected neutrophils, (d) NS88.2rep infected neutrophils and (e) NS88.2 infected neutrophils at 30 min. (f) Neutrophils were sampled over 180 min and the percentage of viable (AV−Z−) neutrophils are shown. Results are pooled means ± SD (n = 3 donors). Linear mixed model “p” values for treatment and time are stated. *p < 0.05, ***p < 0.001 and ****p < 0.0001, black denotes significance from control and grey denotes between NS88.2rep and NS88.2.
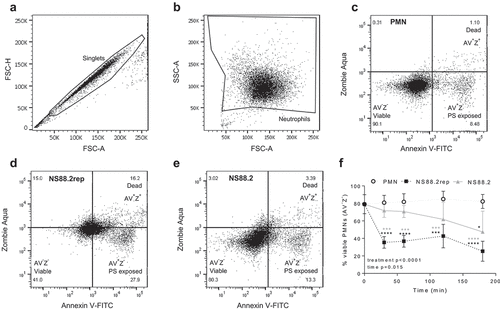
Figure 3. GAS infection increases inflammatory caspase abundance and activation in human neutrophils and induces IL-1β and TNF-α release. (a) Human neutrophil (PMN) lysates uninfected and (30, 60 and 180 min) during GAS infection immunoblotted for caspase-3, caspase-8, caspase-1, caspase-4 and GAPDH. Sample images from a single donor are displayed and represent triplicate experiments (Figures S3 and S4). SE=short exposure, LE=long exposure. Bands were quantified (ImageJ) and normalised against total protein (Figure S2). (b) Neutrophil inflammatory caspase activation during GAS infection using FLICA (FAM-YVAD-FMK) via flow cytometry (n = 3 donors). Singlets/PMNs (Figures S5A and B) and FAM-FLICA fluorescence (Figure S5C) was used for gating. Neutrophil cytokine release in the presence or absence of GAS was measured (0, 60, 180 and 360 min) using the LEGENDplex™ human inflammation cytometric bead assay. Neutrophils differentially released (c) IL-1β, (d) TNF-α and (e) IL-8 in response to GAS infection (duplicate measurements, n = 6 donors). Results are the pooled means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
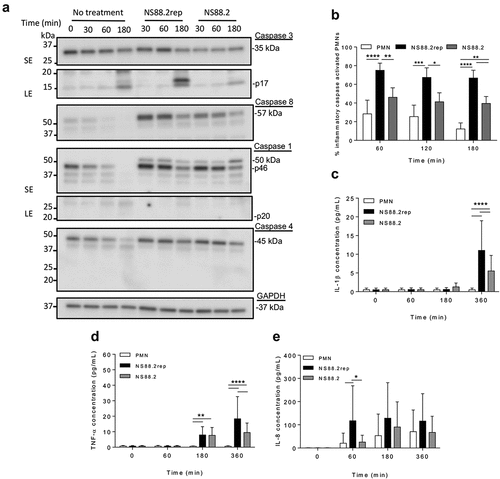
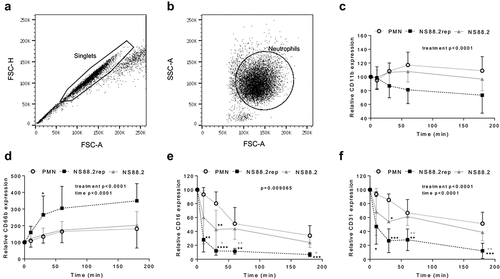
Figure 4. GAS infection influences neutrophil functionality. Human neutrophils (PMNs) were incubated in the absence or presence of GAS and cell-surface CD expression assessed over 180 min by flow cytometry. Neutrophils were sequentially gated as (a) singlets then (B) viable neutrophils, where plots (a-b) are representative and show NS88.2 infected neutrophils at 30 min. Neutrophil (c) CD11b-FITC (n = 4 donors), (d) CD66b-PerCP/Cy5.5 (n = 5 donors), (e) CD16-FITC (n = 4 donors) and (F) CD31-PE/Cy7 (n = 4 donors) was quantified for each donor as relative mean fluorescence intensity. Results are the pooled means ± SD. Linear mixed model “p” values represent interaction (treatment*time) or are stated. *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001, with black denoting significance from control and grey between NS88.2rep and NS88.2.
Supplemental Material
Download MS Word (8.1 MB)Data availability statement
The raw data supporting the conclusions of this article are not freely available due to limits of ethics approval, however project requests can be made by contacting the corresponding author for access and amendments on a case-by-case basis.
