Figures & data
Figure 1. (a) Schematic diagram of phenuivirus structure and (b) transmission cycle of phenuivirus. (a) Phenuvirus comprises three RNA segments large (L) segment, medium (M) segment, and small (S) segment, which encodes RNA-dependent RNA polymerase (RdRp), glycoprotein, non-structural (NS) protein, and nucleoprotein (NP), respectively. The L and M segments are encoded in a negative-sense orientation, whereas the S segment is encoded in an ambisense orientation. Additionally, the table in the left notes the length of each segment for RVFV, SFTSV, HRTV, and GTV.
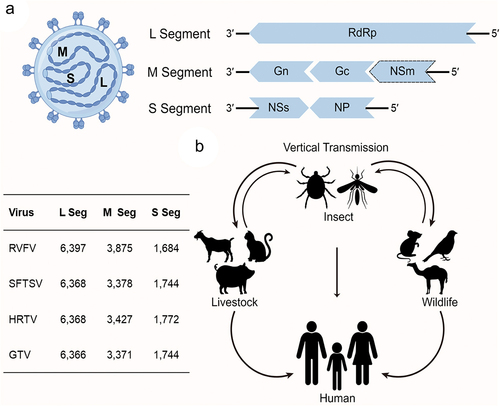
Figure 2. Phenuivirus protein components antagonize the pattern recognition receptor induced antiviral effect. Phenuivirus structure or non-structure protein can directly target multiple antiviral proteins for immune escape, including cGAS, STING, TRIM25, MAVS, TBK1, etc.
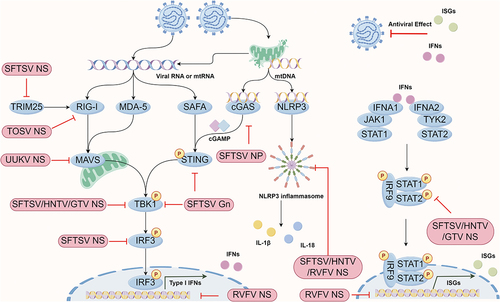
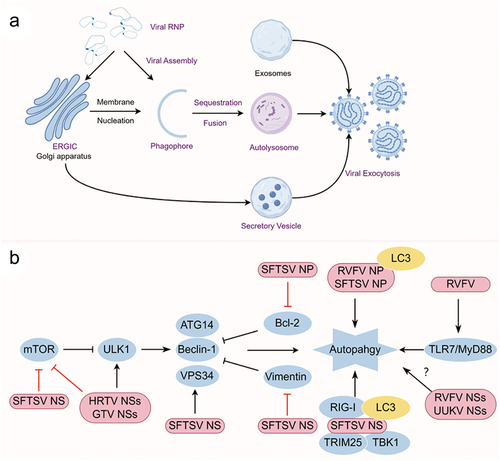
Figure 3. (a) Schematic presentation of the phenuivirus replication cycle and (b) autophagy induction pattern of phenuivirus. (a) Phenuivirus glycoprotein (GP) is synthesized by membrane-bound ribosomes at the ER and subsequently translocated to the ERGIC and Golgi complex. GP is anticipated to recruit the RNP complex for viral assembly and maturation. Subsequently, mature virus particles are released from the cell via secretory vesicles or autophagic vesicles. Alternatively, CD63-positive exosomes also facilitate the exocytosis of phenuivirus in a receptor-independent manner. (b) SFTSV NP induces classical autophagy by disrupting the association between beclin-1 and BCL2. SFTSV NSs induces classical autophagy through several mechanisms, including sequestering mTOR into IBs, promoting vimentin degradation via K48-linked ubiquitin-proteasome pathway, or facilitating the formation of beclin-1-dependent autophagy initiation complexes. Notably, IBs induced by SFTSV NSs may function as autophagy-like vesicles that sequester antiviral proteins such as TBK1, thereby promoting TBK1 degradation for immune evasion. Additionally, RVFV NP interacts with p62 and LC3 to induce autophagy and subsequently inhibit the antiviral innate immune response.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
