Figures & data
Figure 1. Overview of cilium structure, function and disease associations. (A) Schematic of cilium structure. TZ; transition zone, Ax; axoneme, DA; distal appendages, sDA; subdistal appendages; MT; microtubules, CP; ciliary pocket. (B) Epifluorescence image of a hTERT-RPE1 cell expressing an ARL13B-GFP reporter that stains the ciliary membrane (green). Nucleus stained blue (DAPI). Dotted line denotes the plasma membrane. Scale bar; 5 μm. (C) SEM image of a kidney epithelial cell cilium. Image provided by K. Phelps (Electron Microscopy Unit, UT Southwestern Medical Center). Scale bar; 1 μm. (D) TEM image of the proximal end of a primary cilium on a hTERT-RPE1 cell. Scale bar; 200 nm. (E) TEM cross section of a C. elegans sensory cilium showing the 9 doublet microtubules (plus additional inner singlet microtubules). Scale bar; 100 nm. (F) Selection of ciliary-based functions. (G) Ciliopathies and associated phenotypes.
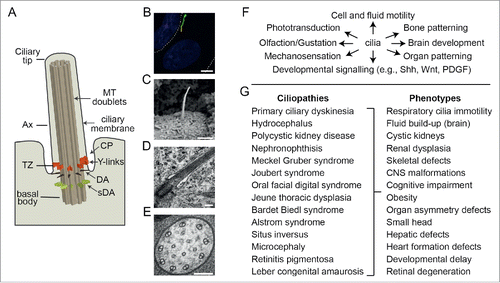
Figure 2. Overview of cilia-related transport functions for Rab proteins. Shown are Rab and Rab-like proteins mapped to major routes of ciliary membrane protein (red) trafficking and IFT (intraflagellar transport) machinery. A; IFT-A complex, B; IFT-B complex, RE; recycling endosome, EE; early endosome. IFT motors (kinesin-2 and IFT-dynein) shown in blue.
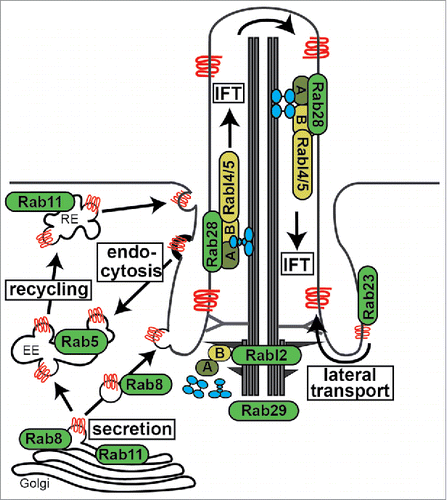
Figure 3. RAB cascades during ciliogenesis and protein transport. (A) Proposed model of RAB11-RAB8 cascade during intracellular ciliogenesis. Shortly after induction of ciliogenesis, Rab11 activates and recruits Rabin8 to the pericentriolar compartment and vesicles (V) dock to the distal appendages of the mother centriole forming small ciliary vesicles (CV). EHD1 together with SNAP29 promotes the fusion of these small vesicles resulting in the maturation of a large CV that caps the distal end of the mother centriole. Rab8, which is recruited and activated by Rabin8, subsequently drives CV membrane extension, and IFT elongates the ciliary axoneme (Ax). This is followed by CV fusion with the plasma membrane (PM), often resulting in the formation of a ciliary pocket (CP). (B) ARF4-RAB11-RAB8 cascade that sorts and delivers rhodopsin transport carriers (RTC) to the periciliary membrane of photoreceptor cells. TGN; trans-Golgi network.
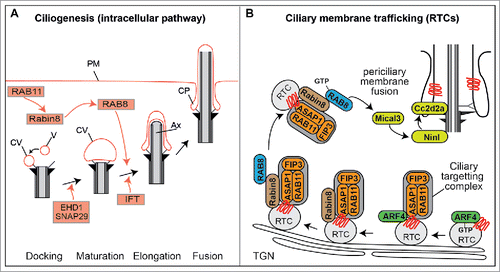
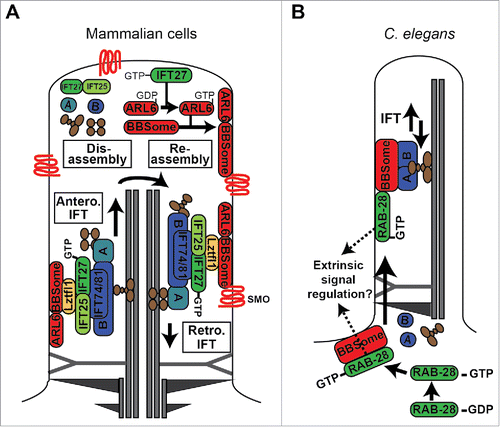
Figure 4. Models of RABL4 (IFT27) and RAB28 association with IFT and the BBSome cargo adaptor. (A) IFT27 forms a submodule of IFT-B that associates with the BBSome via Ltzfl1. At the ciliary tip, where IFT trains are remodelled for retrograde transport, IFT27 acts as a GEF to activate ARL6, which in turn directs the BBSome to the ciliary membrane for assembly into retrograde IFT trains that remove signaling proteins such as Smoothened (Smo) from the cilium. IFT complexes (A and B) shown in blue. IFT motors (kinesin-II and cytoplasmic dynein) shown in brown. (B) In C. elegans, activated RAB-28 is targeted to the periciliary membrane via the BBSome where it incorporates into IFT trains as cargo. RAB-28 is proposed to serve cell non-autonomous functions by regulating extrinsic signaling to a nearby glial cell.Citation164 Note that it remains to be shown if RAB-28 directly interacts with the BBSome. For simplicity, only one microtubule doublet is shown in the cartoon.