Figures & data
Figure 1. Rab3a silencing significantly reduces the MLV Gag protein level. (a) Endogenous Rab3a levels in the empty or shRab3a-encoding lentiviral vector-transduced 293 T cells were analysed by western blotting. (b) Rab3a-silenced cells were transfected with amphotropic MLV vector construction plasmids. Transduction titres of culture supernatants obtained from the transfected cells were measured. Transduction titres of culture supernatants obtained from the empty vector-transduced cells were always set to 1. Relative values of transduction titres in the empty vector-transduced cells ± standard deviations (SD) are indicated. This experiment was repeated three times. Asterisks indicate statistically significant differences. (c) Cell lysates and virion fractions prepared from the transfected cells were analysed by western blotting using the antiMLV Gag p30 antibody (upper panel). Levels of p30 were normalized by actin. The normalized p30 levels in the empty vector-transduced cells were always set to 1. Relative values to the normalized p30 levels in the empty vector-transduced cells ± SD are indicated (lower panel). This experiment was repeated three times. (d) Replication-competent Moloney MLV-producing TE671-mCAT1 cells were transduced with empty or shRab3a-encoding lentiviral vector. MLV Gag p30 and Rab3a levels in the transduced cells were analysed by western blotting. (e) Viral titres of the culture supernatants obtained from the transduced cells are indicated. This experiment was repeated three times. (f) Human 293 T cells transduced with empty or shRab3a-encoding lentiviral vector were inoculated with an amphotropic MLV vector. Transduction titres of empty vector-transduced cells were always set to 1. Relative values of transduction titres ± SD are indicated. This experiment was repeated three times.
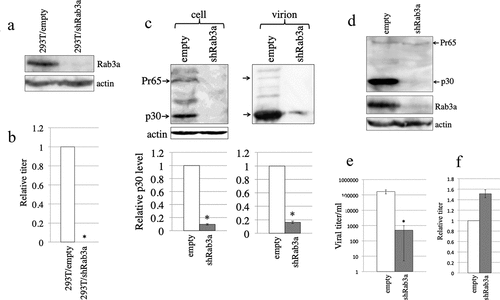
Figure 2. Rab3a is required for MLV Gag protein expression. (a) Control and Rab3a-silenced 293 T cells were transfected with amphotropic MLV vector construction plasmids together with pcDNA3.1 or Rab3a RS-HA expression plasmid. Cell lysates prepared from the transfected cells were analysed by western blotting using the antiMLV Gag p30, antiHA, or antiactin antibody. This experiment was repeated two times. (b) Transduction titres of the culture supernatants obtained from the transfected cells were measured. The transduction titres of the control cells transfected with pcDNA3.1 were always set to 1. Relative values to the transduction titres of the control cells transfected with pcDNA3.1 ± SD are indicated. Single asterisks indicate significant differences compared with the titres of the control cells transfected with pcDNA3.1. Double asterisks indicate significant differences compared with the titres of Rab3a-silenced cells transfected with cDNA3.1. This experiment was repeated three times.
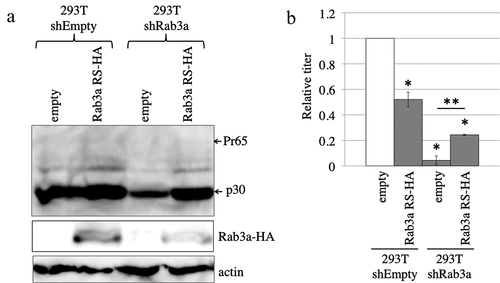
Figure 3. The MLV Gag protein is degraded in the lysosomes of Rab3a-silenced cells. (a) Control and Rab3a-silenced 293 T cells were transfected with the MLV Gag-Pol expression plasmid and treated with DMSO, concanamycin A (CMA), or MG-132. Cell lysates prepared from the treated cells were analysed by western blotting using the antiMLV Gag p30 or antiactin antibody. (b) Band intensities of MLV Gag p30 and actin proteins were measured using a densitometer, and p30 levels were normalized by actin levels. Normalized p30 levels of the control cells treated with DMSO were always set to 1. Relative values to the normalized p30 levels of the control cells treated with DMSO ± SD are indicated. Single asterisks represent significant differences compared with the p30 levels of the control cells treated with DMSO. double asterisks indicate significant differences compared with the p30 levels of the Rab3a-silenced cells treated with DMSO. This experiment was repeated three times. (c) Control 293 T cells were transfected with the MLV Gag-Pol expression plasmid and treated with DMSO, CMA, or MG-132. Cell lysates prepared from the treated cells were analysed by western blotting using the antiMLV Gag p30 or antiactin antibody. (d) Band intensities of the MLV Gag p30 and actin proteins were measured using a densitometer, and the p30 levels were normalized by actin levels. The normalized p30 levels of the control cells treated with DMSO were always set to 1. Relative values to the normalized p30 levels of the control cells treated with DMSO ± SD are indicated. This experiment was repeated three times.
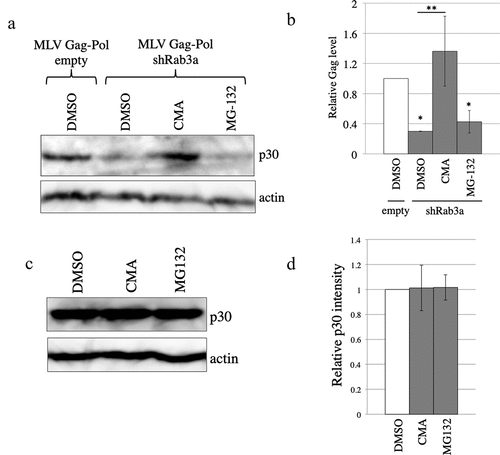
Figure 4. The MLV Gag protein inhibits the complex formation of CD63 and Rab3a. (a) Human 293 T cells were transfected with Rab3a WT-HA, T36N-HA, or Q81L-HA expression plasmid together with pcDNA3.1 or MLV Gag-Pol expression plasmid. Cell lysates prepared from the transfected cells were immunoprecipitated with the antiMLV p30 antibody. The precipitates were analysed by western blotting using the antiHA or antiMLV p30 antibody (left panels). Western blots of the cell lysates obtained using the antiMLV p30, antiHA, or antiactin antibody are also shown in the right panels. (b) Human 293 T cells were transfected with various combinations of pcDNA3.1, Rab3a WT-HA, CD63-GFP, and MLV Gag-Pol expression plasmids. Cell lysates prepared from the transfected cells were immunoprecipitated using the antiGFP antibody. The precipitates were analysed by western blotting using the antiHA or antiGFP antibody (left panels). Western blotting of cell lysates was performed using the antiHA, antiGFP, antiMLV p30, or antiactin antibody (right panels). These experiments were repeated two times.
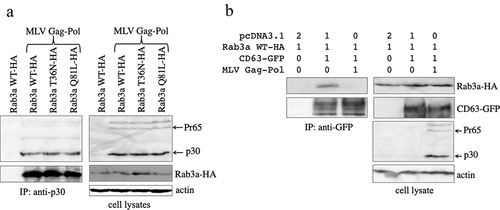
Figure 5. N-terminally HA-tagged Rab3a stabilizes and binds to the MLV Gag protein. (a) Human 293 T cells transduced with empty or shRab3a-encoding lentiviral vector were transfected with amphotropic MLV vector construction plasmids. Cell lysates prepared from the transfected cells were analysed by western blotting using antiMLV p30, antiHA, or antiactin antibody. This experiment was repeated two times. (b) Transduction titres of culture supernatants obtained from the transfected cells were measured. Transduction titres of control cells transfected with pcDNA3.1 were always set to 1. Relative values to transduction titres of control cells ± SD are indicated. This experiment was repeated three times. Single asterisks show significant differences compared with the transduction titres of the control cells. Double asterisks indicate significant differences between the two indicated groups. (c) Human 293 T cells were transfected with the indicated expression plasmids. Cell lysates prepared from the transfected cells were immunoprecipitated using the antiMLV p30 antibody. The precipitates were analysed by western blotting using the antiHA or antiMLV p30 antibody (left panels). The cell lysates were analysed by western blotting using the same antibody (right panels). This experiment was repeated two times.
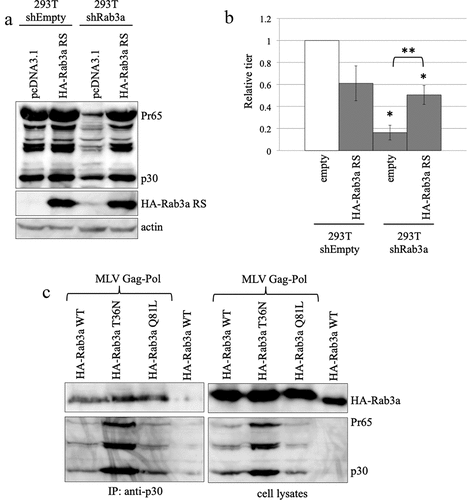
Figure 6. Active and inactive forms of C-terminally HA-tagged Rab3a decrease and increase MLV vector infectivity, respectively. (a) Human 293 T cells were transfected with amphotropic MLV vector construction plasmids together with pcDNA3.1, Rab3a WT-HA, T36N-HA, or Q81L-HA expression plasmids. Culture supernatants of the transfected cells were inoculated into TE671 cells, and transduction titres were measured. The transduction titres of the pcDNA3.1-transfected cells were always set to 1. Relative values to the transduction titres of the pcDNA3.1-transfected cells ± SD are indicated. This experiment was repeated three times. Asterisks indicate significant differences compared with control cell titres. (b) Cell lysates and virion pellets from the transfected cells were analysed by western immunoblotting. (c) p30 levels in virion pellets normalized by p30 levels in the cell lysates were calculated. Normalized p30 levels in the virion pellets of the pcDNA3.1-transfected cells were always set to 1, and relative values ± SD are indicated. This experiment was repeated three times.
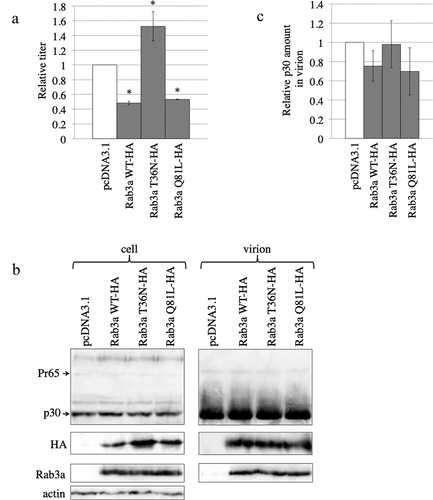
Figure 7. Active and inactive forms of N-terminally HA-tagged Rab3a decrease and increase MLV Gag protein levels, respectively. (a) Human 293 T cells were transfected with the MLV Gag-Pol expression plasmid together with HA-Rab3a WT, T36N, or Q81L expression plasmid. The transfected cells were treated with methanol and then with mouse antiHA and goat antiMLV p30 antibodies. Then, the cells were treated with Cy3-conjugated antimouse IgG (red) and FITC-conjugated antigoat antibodies (green). The treated cells were observed by confocal microscopy. (b) Human 293 T cells were transfected with amphotropic MLV vector construction plasmids together with pcDNA3.1, HA-Rab3a WT, T36N, or Q81L expression plasmids. Transduction titres of culture supernatants obtained from the transfected cells were measured. The transduction titres of the pcDNA3.1-transfected cells were always set to 1. relative values to the transduction titres of the pcDNA3.1-transfected cells ± SD are indicated. This experiment was repeated three times. Asterisks indicate significant differences compared with control cell titres. (c) Cell lysates prepared from the transfected cells were analysed by western blotting. (d) p30 levels normalized by the p30 levels in the cell lysates and virion fractions were calculated. Normalized p30 levels in the pcDNA3.1-transfected cells were always set to 1, and relative values ± SD are indicated. This experiment was repeated three times.
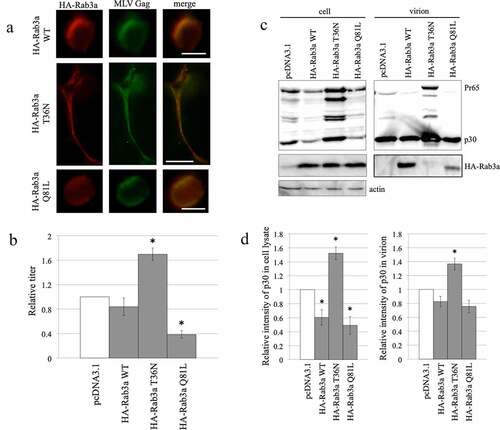
Figure 8. Mouse Rab3a stabilizes the MLV Gag protein. (a) Human 293 T cells were transfected with the mRab3a RS-HA expression plasmid together with the pcDNA3.1 or MLV Gag-Pol expression plasmid. The MLV Gag protein was precipitated by the antip30 antibody. The precipitates were analysed by western immunoblotting using the antiHA antibody (upper panel). Cell lysates prepared from the transfected cells were analysed by western blotting (lower panel). This experiment was repeated two times. (b) Control and Rab3a-silenced 293 T cells were transfected with amphotropic MLV vector construction plasmids together with pcDNA3.1 or mRab3a RS-HA expression plasmid. Culture supernatants of the transfected cells were inoculated into 293 T cells, and transduction titres were measured. The transduction titres of the control cells were always set to 1, and relative values ± SD are indicated. Single asterisks indicate significant differences compared with control cell titres. Double asterisks represent significant differences between the two indicated groups. This experiment was repeated three times. (c) Cell lysates prepared from the transfected cells were analysed by western immunoblotting. This experiment was repeated two times.
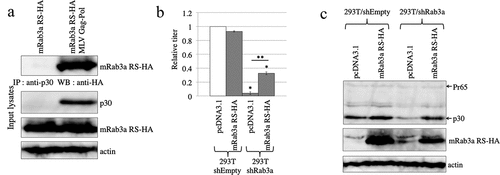
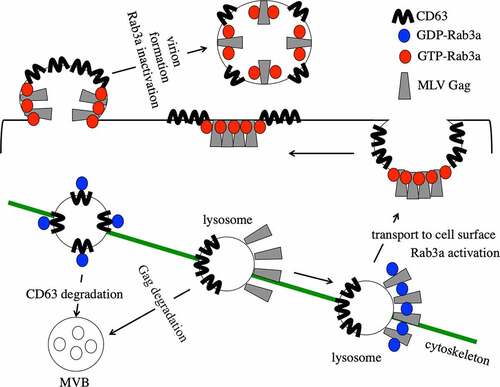
Figure 9. MLV inhibits Rab3a-mediated degradation of CD63. (a) Human 293 T cells were transfected with C-terminally GFP-tagged CD63 expression plasmid together with pcDNA3.1 or amphotropic MLV vector construction plasmids. Cell lysates and virion fractions were prepared from the transfected cells. Virion fractions were collected by centrifugation through 20% sucrose. The cell lysates and virion fractions were analysed by western blotting. This experiment was repeated two times. (b) Human 293 T cells were transfected with Rab3a WT-HA and CD63-GFP expression plasmids with or without the MLV Gag-Pol expression plasmid. Cell lysates prepared from the transfected cells were analysed by western blotting using the antiGFP, antiHA, antiMLV Gag p30, or antiactin antibody. This experiment was repeated two times. (c) Cell lysates prepared from replication-competent Moloney MLV-producing and uninfected TE671-mCAT1 cells were analysed by western blotting using the antiMLV p30, antiCD63, or antiactin antibody (left panel). CD63 levels normalized by actin levels were calculated. The normalized CD63 levels in uninfected cells were always set to 1, and relative values are indicated (right panel). Asterisks indicate significant differences. this experiment was repeated three times.
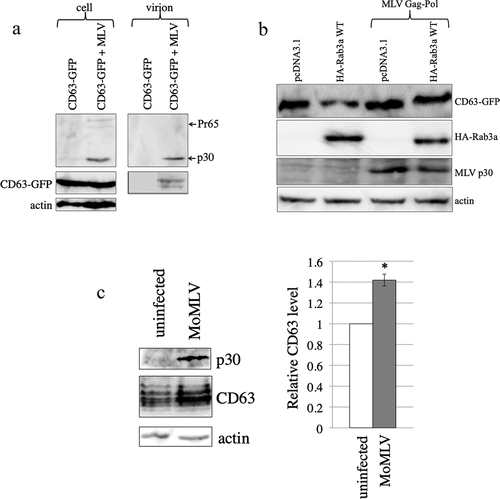
Figure 10. Exogenous CD63 expression has no effect on MLV virion production and infectivity. (a) Human 293 T cells were transfected with amphotropic MLV vector construction plasmids together with an empty or CD63-GFP expression plasmid. Transduction titres of the culture supernatants obtained from the transfected cells were measured in TE671 cells. Transduction titres of the empty plasmid-transfected cells were always set to 1. Relative values to the transduction titres of the empty plasmid-transfected cells ± SD are indicated. This experiment was repeated three times. (b) Cell lysates and virion fractions prepared from transfected cells were analysed by western blotting. This experiment was repeated two times.
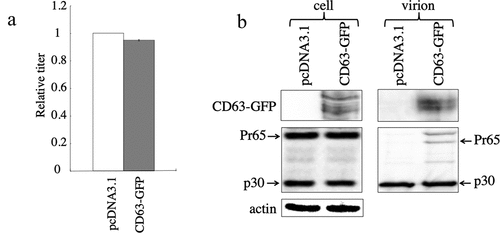
Figure 11. CD63 silencing inhibits the infectivity of released MLV particles. (a) Control and shCD63-expressing TE671 cells were transfected with ecotropic MLV vector construction plasmids. Transduction titres of the culture supernatants obtained from the transfected cells were measured. The transduction titres of the control cells were always set to 1. Relative values to control cell transduction titres ± SD are indicated. Asterisks indicate statistically significant differences. This experiment was repeated three times. (b) Cell lysates and virion fractions from the transfected cells were analysed by western blotting. This experiment was repeated two times. (c) Replication-competent Moloney MLV-producing cells were transduced with the shCD63-expressing lentiviral vector. Viral titres of culture supernatants obtained from empty or shCD63-transduced cells were measured. This experiment was repeated three times. (d) Cell lysates and virion fractions from the transduced cells were analysed by western blotting. This experiment was repeated two times. (e) Human 293 T cells transduced with empty or shCD63-encoding lentiviral vector were inoculated with an amphotropic MLV vector, and transduction titres were measured. Transduction titres of empty vector-transduced were always set to 1. Relative values to control cell transduction titres ± SD are indicated. This experiment was repeated three times.
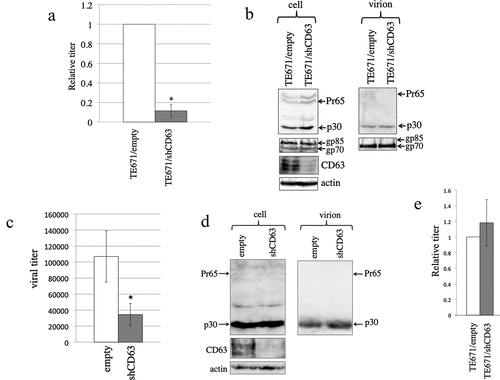
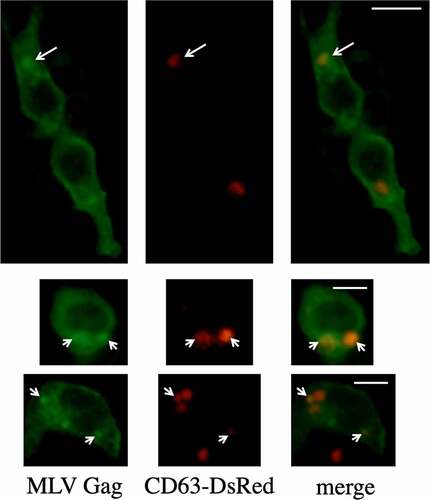
Figure 12. CD63 is colocalized with the MLV gag protein. Human 293 T cells were transfected with MLV Gag-Pol and CD63-DsRed expression plasmids and then permeabilized with methanol. The cells were treated with the goat antiMLV p30 antibody and then with the FITC-conjugated antigoat IgG antibody. The cells were observed under a confocal microscope. Arrows indicate colocalization of the MLV Gag and CD63-DsRed proteins. Scale bar, 10 µm.