Figures & data
Figure 1. Comparative analysis of breast cancer tissue specimens upon culture in static conditions or under perfusion. (A) Fragments from a representative surgically excised breast cancer specimen were cultured for the indicated time points in static or perfused culture conditions. Samples were then fixed, paraffin embedded, and sections were HE stained (Magnification 100× (Static) and 200× (Perfusion)). (B) Comparative analysis of percentages of vtc in fragments from at least seven different breast cancer surgical specimens cultured in static or perfused conditions for the indicated time points (*p ≤ 0.05; **p ≤ 0.01).
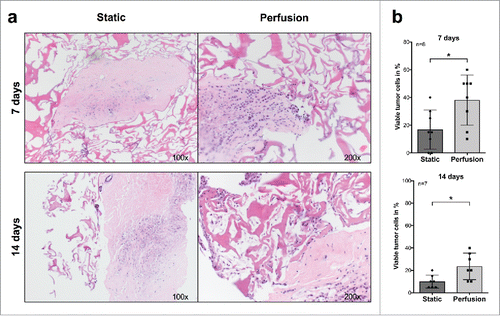
Table 1. Clinico-pathological parameters of all included breast cancer patients.
Figure 2. Culture of breast cancer fragments in perfused bioreactors: preservation of TME cellular components. (A) Fragments from two representative surgically excised breast cancer specimens were inserted between collagen type I scaffolds and cultured in perfused bioreactors for the indicated time. Samples were then collected, fixed, and paraffin embedded. HE-stained sections from the original surgical specimens, cultured fragments and infiltrated scaffolds were then comparatively evaluated (Magnification 100× or 200× as indicated). (B) Quantitative analysis of tumor cell and lymphocyte numbers within cultured tumor fragments and collagen scaffolds at the indicated time points. Displayed results summarize data from 27 different breast cancer specimens. In one cultured specimen, the initial tissue fragment did not contain cancer, and in two additional specimens, counting of tumor cells and lymphocytes could not be performed due to lack of material, and thus these specimens were omitted for data analysis. Non-parametric Mann–Whitney test (****p ≤ 0.0001).
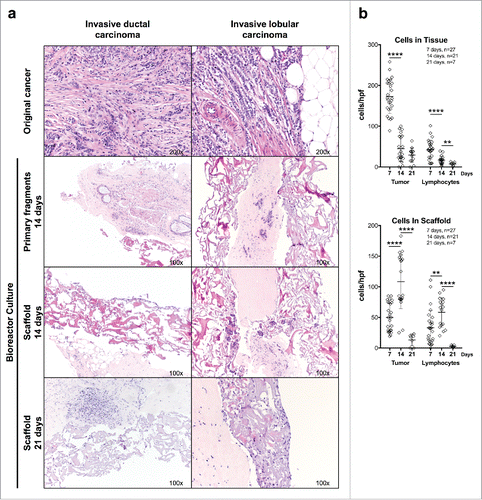
Figure 3. Immunohistochemical evaluation of breast cancer tissue fragments cultured in perfused bioreactors. (A) Fragments from the indicated representative surgically excised breast cancer specimen were cultured for 14 d in a perfused bioreactor. Samples were then collected fixed and paraffin embedded. Sections were stained with serological reagents recognizing the indicated markers (Magnification 200×). (B) Quantitative analysis of numbers of cells expressing the indicated markers in fragments from one breast cancer sample cultured for the indicated time points. The low lymphocyte count in the treated sample on day 7 is most probably due to tumor heterogeneity.
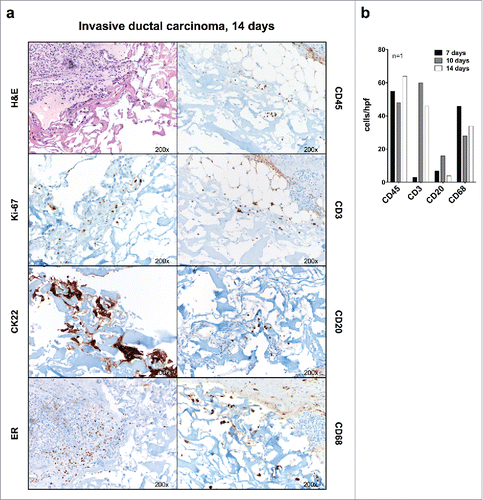
Figure 4. Effects of anti-estrogen treatment on ER+ breast cancer fragments cultured in perfused bioreactors. (A) Fragments from a representative ER+ surgically excised breast cancer were cultured for 21 d in the presence or absence of Fulvestrant (100 nM). Samples were then fixed and paraffin embedded, sections were stained with HE (upper 2 and central 2 panels, magnification 100× and 200×, respectively) or immunohistochemically stained by using a CK22 specific reagent (lower two panels) (Magnification 200×). (B) Cumulative analysis of vtc per high-power field in fragments from six different ER+ breast cancers following culture in perfused conditions in the presence or absence of Fulvestrant. Data represented as ratio compared with day 0 (*p ≤ 0.05).
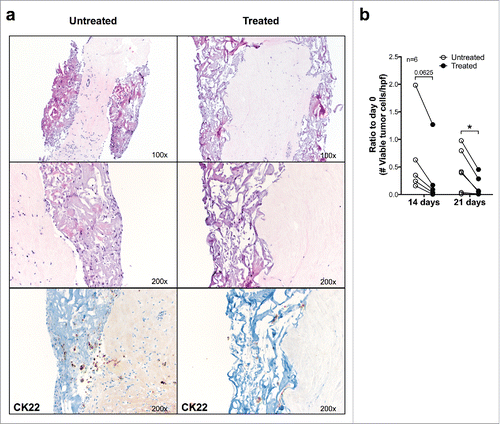
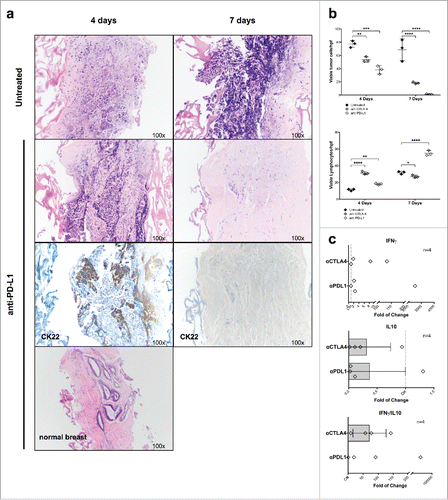
Figure 5. Culture of breast cancers in the presence of therapeutic mAb recognizing immunological checkpoints. (A) Fragments from a representative, untreated TNB cancer were cultured for the indicated time points in perfused bioreactors in the presence or absence of anti-PD-L1 therapeutic mAb (10 μg/mL). Samples were then fixed and paraffin embedded. Sections were stained with HE- and anti CK22-specific reagents (Magnification 100×). (B) Fragments from three different untreated TNB cancers were cultured in perfused bioreactors in the presence or absence of anti PD-L1 or anti CTLA-4 therapeutic mAbs (10 μg/mL) for the indicated time points. Numbers of vtc and lymphocytes in cultured tissue were then comparatively evaluated. Two-way ANOVA, matched values with Dunnett's multiple comparison test (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001). (C) Triplicate fragments of a luminal A breast cancer were treated for 7 d in perfused bioreactors, in the presence of the indicated therapeutic mAbs. Total cellular RNA was then extracted and reverse transcribed. The expression of the indicated genes was evaluated by qRT-PCR. Data are reported as fold increases or decreases, as compared with the expression of the same genes in untreated samples. IFNγ/IL-10 gene expression ratios are also shown.