Figures & data
Table 1 Demographic and baseline characteristics
Table 2 Adverse events following a single oral dose of HMS5552 in healthy subjects
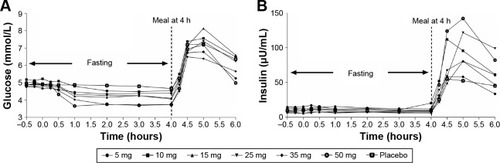
Table 3 Summary of geometric mean (SD) pharmacokinetic parameters of HMS5552
Figure 1 Mean HMS5552 plasma concentration (ng/mL) versus time after a single oral administration in healthy subjects at six different doses (n=8 per dose group).
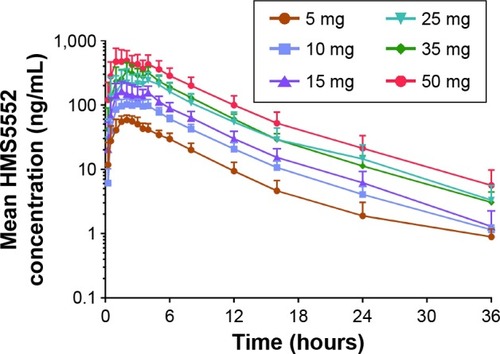
Figure 2 HMS5552 and its major metabolites in human plasma and urine.
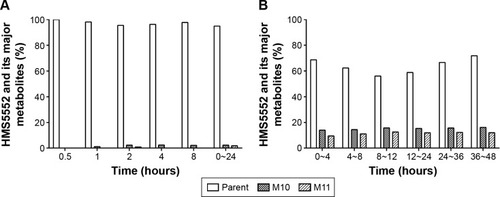
Figure 3 Effect of HMS5552 on mean glucose and insulin concentrations during fasting and after a standardized meal.