Figures & data
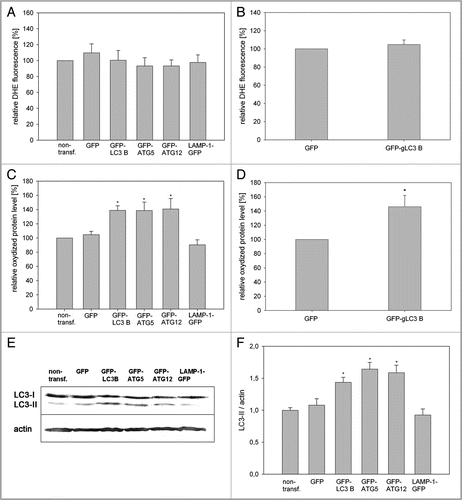
Figure 1 Mitochondrial dynamics does not act in early quality control after ROS-induced mitochondrial damage. (A) Young HUVEC were treated for 10 min with hydrogen peroxide (final concentration 13.2 mM) or were stained with MTR and irradiated for 15 or 30 min. Immediately after treatment the amount of oxidized proteins in mitochondrial and whole cell lysates was analyzed by oxyblotting. After irradiation, mitochondrial lysates contained a significantly higher amount of oxidized proteins compared with whole cell lysates. In contrast, hydrogen peroxide-treated cells showed a much higher content of oxidized proteins in the whole cell lysate compared with the mitochondrial fraction; n = 3; p < 0.05. (B) Mitochondria in HeLa cells transfected with mtCFP were stained with MTR and either irradiated or not treated, subsequently co-cultured with nonirradiated mtGFP transfected HeLa cells and after 4 h fused by PEG. Cycloheximide (CHX) was added 30 min before PEG fusion to prevent the influence of ongoing protein synthesis. Samples were fixed at the indicated time points after PEG treatment and depicted by CLSM. While in controls complete mixing of the fluorescent proteins was observed, no fusion of fragmented mitochondria in irradiated cells took place. Color coding: green, GFP; blue, CFP; red, MTR; magenta, GFP, CFP and MTR; white, MTR plus CFP; yellow, MTR and GFP. (C) The PEG fusion experiments were quantified by Pearson correlation. A Pearson correlation factor of 1 means 100% colocalization, while low and negative values stand for lack of colocalization and strict separation, respectively. In control cells, an almost complete colocalization of mtCEF/MTR and mtGFP was observed while no colocalization of mtCEF/MTR and mtGFP was detected in the irradiated samples; n = 5−11; p < 0.05.
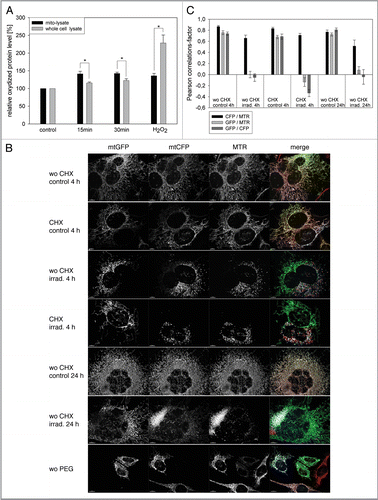
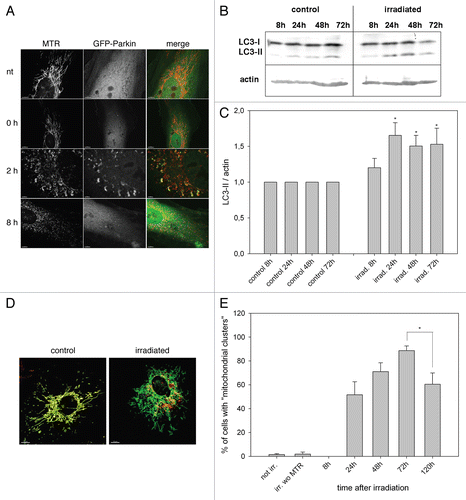
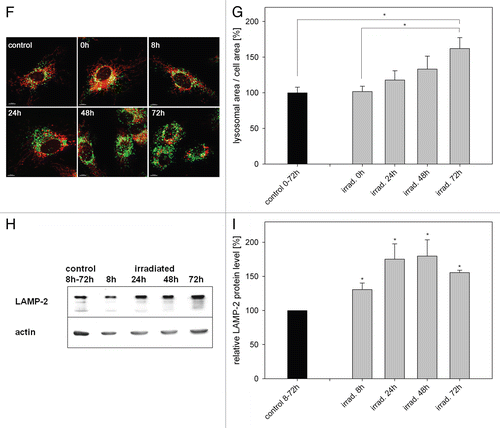
Figure 3 Overexpression of ATG5, LC3B and ATG12 increased life span in two cell models. (A) Young HUVEC were stained with MTR and irradiated (irrad) or nonirradiated (control). At the indicated time points after irradiation, mRNA levels were quantified by semiquantitative RT-PCR and normalized to the nonirradiated control. PGC1α, ATG5, ATG12 and LC3B were upregulated 8 h and 72 h after irradiation; n = 3−6; p < 0.05. (B) MTR-stained HeLa cells were transfected with plasmids expressing GFP-LC3B, GFP-ATG5 and GFP as control. 48 h after transfection the MTR fluorescence was quantified. The MTR fluorescence of GFP-LC3B and GFP-ATG5 was significantly decreased compared with GFP-expressing cells; n = 3; p < 0.05. (C) HUVEC stably transfected with GFP, GFP-ATG5, GFP-LC3B, GFP-ATG12 and LAMP-1-GFP were stained with MTR and analyzed by CLSM. In LC3B-, ATG5-and to a lesser amount ATG12-overexpressing senescent cells, a cytosolic GFP fluorescence with fluorescent autophagosomes was discernible; in LAMP-1 transfected cells the lysosomes are clearly labeled. (D) Young HUVEC (PD13) that were either nontransfected or stably transfected with GFP, GFP-ATG5, GFP-LC3B GFP-ATG12 and LAMP-1-GFP were cultivated in triplicate until they reached replicative senescence. All autophagy protein-expressing cells exhibited a significantly extended life span and higher PD numbers compared with control cells (non-transfected and GFP-overexpressing cells). In contrast LAMP-1-overexpressing HUVEC reached senescence after a similar cultivation time as the control cells, indicating that enhanced expression of autophagy proteins prolonged the replicative life span of HUVEC; p < 0.05. (E) Young CEF in passage 8 were infected with virus coding either for GFP or GFP-gLC3B (see inset) and cultivated in triplicate until they reached replicative senescence. Overexpression of GFP-gLC3B resulted in a prolonged replicative life span and significantly increased PD number in comparison to GFP-transfected cells, in accordance with the results obtained for HUVEC presented in ;p < 0.005. (F) Pre-senescent HUVEC (PD 22) were either nontransfected or stably transfected with plasmids expressing GFP, GFP-ATG5, GFP-LC3B GFP-ATG12 or LAMP-1-GFP. Cells were cultivated in triplicate until they reached replicative senescence. In contrast to young HUVEC (), no expansion of the life span or the PD number became apparent, indicating the absence of a rejuvenating effect.
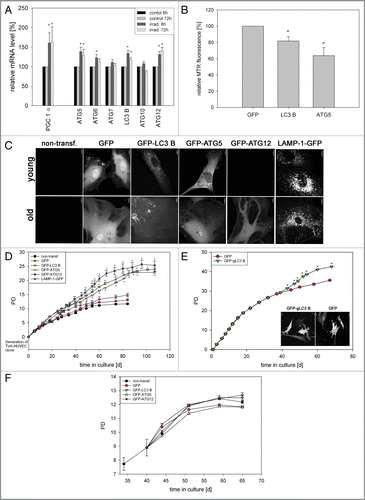
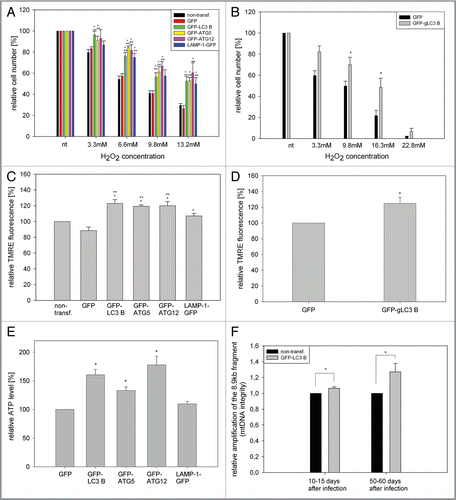
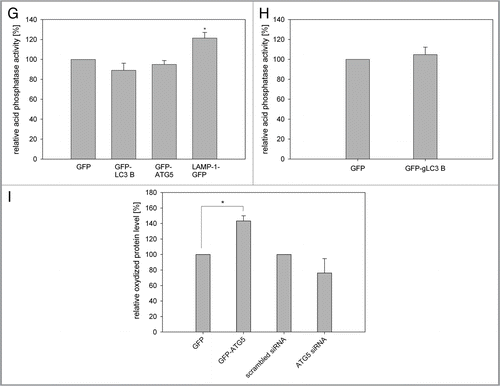
Figure 4 Overexpression of LC3B, ATG5, ATG12 and LAMP-1 mediates cell protective effects. (A and B) Young HUVEC expressing the indicated GFP fusion proteins (A) or young CEF overexpressing GFP and GFP-gLC3B (B) were treated with increasing doses of hydrogen peroxide for 10 min and the relative cell number of surviving adherent cells was determined and normalized to the untreated cells 24 h after treatment. Overexpression of the autophagy genes or LAMP-1 protected against hydrogen peroxide-induced cell death; (A) n = 4−6; p < 0.05; * = significant to GFP transfected cells, ** = significant to nontransfected cells; (B) n = 8; p < 0.05. (C and D) Young HUVEC stably overexpressing the indicated proteins (C) and young CEF overexpressing GFP and GFP-gLC3B (D) were stained with the mitochondrial membrane potential indicator TMRE and depicted by microscopy with constant settings. The TMRE fluorescence intensity was normalizedtothe fluorescence intensity of nontransfected HUVEC, and GFP transfected CEF, respectively. Overexpression of the autophagy genes and to a lesser amount LAMP-1 enhanced the mitochondrial membrane potential in both cell models; (C) n = 4−9; p < 0.05, (D) n = 7; p < 0.05. (E) Young HUVEC were stably transfected with GFP, GFP-ATG5, GFP-ATG12, GFP-LC3B and LAMP-1-GFP. Their ATP content was normalized to the GFP-transfected cells. Overexpression of the autophagy genes but not of LAMP-1 enhanced the ATP content; n = 3−4; p < 0.05. (F) A large fragment of the mtDNA (8.9 kb) was amplified by PCR and normalized on an amplified small fragment of the mtDNA (0.2 kb). The relative amplification of the 8.9-kb fragment acts as parameter for mtDNA integrity. The quantification in nontransfected and GFP-LC3B-overexpressing HUVEC at different time points of their growth curve shows an increased relative amplification of the 8.9-kb fragment after LC3B overexpression, indicating enhanced protection against mtDNA damage; 10–15 d (young cells): n = 3; 50–60 d (pre-senescent): n = 6; p < 0.05.