Figures & data
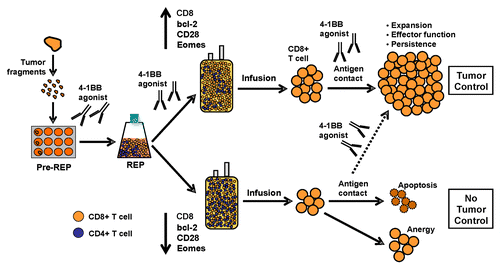
Figure 1. Role for 4–1BB co-stimulation during the expansion of tumor-infiltrating lymphocytes ex vivo and subsequent adoptive cell transfer. We propose that the continuative co-stimulation of tumor-infilrating lymphocytes (TILs) via 4–1BB throughout their expansion ex vivo and upon their reinfusion into patients accelerates the generation of high amounts of CD8+ T cells exhibiting an improved effector-memory profile and increased in vivo persistence. Different stages of TIL expansion—including the initial outgrowth from tumor fragments followed by the rapid expansion protocol (REP) to generate the final infusion product—are schematized. Past expansion protocols (bottom half) have not provided enough co-stimulatory signals, to TILs, in particular through members of the TNF-R family such as 4-1BB resulting in infusion products that are enriched in CD4+ T cells or are generally unable to mediate therapeutic antitumor effects due to poor effector-memory function and in vivo persistence. Conversely, TILs exposed to 4–1BB agonists (top half) maintain the expression of effector-memory markers while upregulating anti-apoptotic proteins and co-stimulatory receptors other than 4–1BB, like CD28. The infusion products generated by this protocol are enriched in CD8+ T cells, have improved survival and expansion capacity in vivo after adoptive transfer, and may exert more robust tumor control.