Figures & data
Figure 1. Basic structures of the group A Paks (Paks 1, 2, and 3), and group B Paks (Paks 4, 5, and 6). Group A Paks have an Autoinhibitory Domain (AID) overlapping the GBD (GTPase Binding Domain), while the Group B Paks have a related sequence adjacent to the GBD.
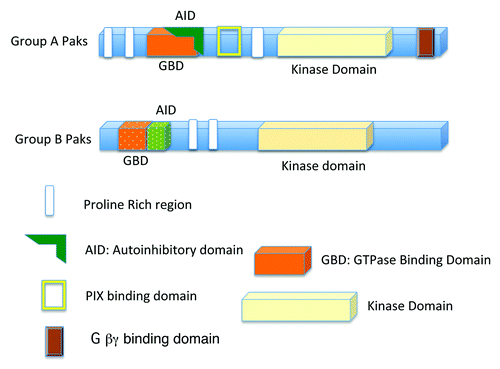
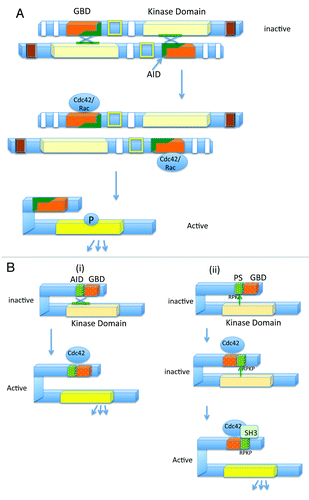
Figure 2. Models representing the activation mechanism of the group A and group B Paks. (A) Activation of the group A Paks: Inactivated group A Paks form dimers, where the AID of one Pak binds the kinase domain of the dimerizing Pak, and inactivates it. Binding to Cdc42 or Rac can relieve this inhibition, resulting in autophosphorylation and kinase activation. (B) Two different models for regulation of the group B Paks: In the first model (i), the AID binds to the kinase domain of the monomeric Pak, in trans, resulting in an inactive conformation. Binding of Cdc42 relieves the inhibition and leads to Pak activation. Unlike the group A Paks, the group B Paks are constitutively phosphorylated, but the kinase takes on an active conformation upon Cdc42 binding. In the second model (ii), the autoinhibitory pseudosubstrate (PS) containing the sequence RPKP is recognized by the kinase domain. This interaction inhibits Pak kinase activity. Cdc42 binding relocalizes Pak within the cell, and proteins containing SH3 domains, such as Src, subsequently activate Pak by competing with the Pak kinase domain, for interacting with the pseudosubstrate domain (modified from refs. Citation15, Citation16, and Citation24)