Abstract
Ketogenic diets (KD) have received increasing interest in neuro-oncology based on their ability to inhibit glioma growth In Vitro and their established role in medically refractory seizures. This review analyses the methodological aspects of KD treatment alongside standard care for patients with gliomas from a nutritional point of view. A literature search was performed in March 2022 searching PubMed and Scopus. We identified 13 articles including 187 patients with a histological—new or recurrent—diagnosis of glioma and treated by KD during the course of the disease. Dietary treatments were categorized as the classical ketogenic diet (CKD), the Modified Atkins diet (MAD), and the medium-chain triglyceride (MCT) diet. We identified a large variation in dietary characteristics regarding restriction of carbohydrates, ketogenic ratio, and additional dietary support. This striking heterogenicity in the methodological approaches of KD treatments made it problematic to compare effects between the included studies. Therefore, a standardized definition of KD for patients with glioma and a consensus on methodological implementation is needed. It would also be desirable to further investigate to what extent KD treatment can be optimized to secure optimal nutrient status and patient satisfaction.
Introduction
Diffuse gliomas are the most common type of primary intracranial tumors in adult life and constitute approximately 70% of all malignant brain tumors (Citation1, Citation2). According to the 2021 World Health Organization (WHO) classification, diffuse gliomas occur as grades 2–4, based on morphological tumor characteristics in combination with specific molecular aberrations (Citation3). The most frequent and aggressive forms of glioma are IDH (isocitrate dehydrogenase)-wildtype glioblastomas (glioma grade 4) (Citation3). Glioblastomas have an incidence rate of 3.2 per 100,000 inhabitants. Although rare, gliomas cause significant morbidity and mortality in the population (Citation1, Citation4). Standard care of glioma includes surgical resection, followed by radiation therapy and chemotherapy (Citation5). Despite multimodal treatment strategies, adult diffuse gliomas WHO grade 2–4 are incurable (Citation6), and alternative treatments are sought.
In a series of classical experiments, Otto Warburg showed that cancer cells have an increased demand for glucose and increased aerobic glycolysis, with a higher ability to convert glucose to lactate even in presence of oxygen (Citation7). This aerobic glycolysis, also known as the “Warburg effect”, is a feature of glioma cells increasing their need for glucose compared to normal cells. Therefore, restricting the amount of glucose as a way to starve glioma cells is an attractive treatment option (Citation8). In a ketogenic diet (KD) with a restricted intake of carbohydrates and a high intake of fat, fatty acids are metabolized into ketone bodies and used to replace glucose as the major fuel source for the central nervous system. Thus, alongside the current standard treatment of gliomas, the KD has a potential role in cancer targeted therapy (Citation9, Citation10). The KD has proven useful in several degenerative neurological diseases (Citation11), and has an established role in medically refractory epilepsy (Citation12, Citation13), especially in children, with approximately 30–60% achieving seizure reduction by 50% after 6 mo, of treatment (Citation14). Adult patients with drug-resistant epilepsy have reported more than 50% seizure reduction in 49% of patients receiving a classical KD (Citation15). Likewise, reduction of seizure rates up to 85%, with seizure freedom rates of 0–55%, have been reported after three months in children, adolescents, and adults (Citation16).
There are several types of ketogenic dietary treatments, containing varying proportions of macronutrients () (Citation17, Citation18). The most commonly used diet is the classical ketogenic diet (CKD), which consists of a macronutrient ratio of 3–4:1, including 3–4 g of fat to every 1 g of protein and carbohydrate The Modified Atkins diet (MAD) is a high-fat, low carbohydrate therapy with a 1–2:1 ketogenic ratio, containing approximately 50% of fat by weight allowing a free intake of protein, fluids, and calories (Citation19). This diet has been considered preferable for adolescents and young adults, by being easier to follow compared to the CKD (Citation20, Citation21). A third option is a medium-chain triglyceride (MCT) diet, in which the long-chain triglycerides are replaced with medium-chain triglycerides by at least 30% (Citation18, Citation22, Citation23). By shifting to increased intake of MCTs, the ketone synthesis is elevated, and patients can achieve ketosis with less ratio of fat in relation to carbohydrates (Citation24). Finally, the low glycemic index treatment (LGIT) diet, containing roughly 40% fat by weight, is used for the treatment of epilepsy (Citation25) but has not yet been reported in the treatment of brain tumors (Citation18). The KD as a single treatment has been shown beneficial for survival in animal models of glioblastoma, but in combination with current anti-tumor treatments, the effects are suggested to be larger (Citation26).
Table 1. Description of ketogenic dietary therapies commonly used in combination with standard treatment among glioma patients.
Previous reviews have focused on the effectiveness of the KD as a therapy for malignant gliomas (Citation27, Citation28), analyzed its effect on tumor growth (Citation29, Citation30), and assessed patient survival (Citation31). However, methodological aspects of the KD related to diet such as adherence, anthropometric measurements, and access to dietary consultation have been less studied. Prior to dietary treatment to be used safely in patients with brain tumors, the length and regimen of the KD have to be established (Citation32). This narrative review aimed to analyze different KD treatments alongside standard care for patients with gliomas from a nutritional point of view, with a focus on the methodological aspects of the diet. We discuss the impact of the different approaches on adherence to the KD, and the diet-related metabolic outcomes.
Materials and Methods
We reviewed the literature to present the existing research studies on KD treatments in patients diagnosed with glioma and to assess the different methodological approaches.
Search Strategy
The literature search was performed by an educated librarian, assisted by the author (A.G.), in March 2022, searching two databases: PubMed and Scopus. The literature search consisted of combined synonyms for KD and glioma (). In PubMed, Medical Subject Headings terms were used to select keywords related to the topic of interest and to find relevant keywords and synonyms. Corresponding search terms were used in a literature search performed in Scopus. Although we did not perform a systematic literature review, the PRISMA Statement protocol (Citation33) was used for guidance to systematically assess the identified articles. The protocol consisted of The PRISMA 2020 Checklist (Citation34), a 27-items checklist that was used for screening and selecting eligible articles. Reference lists of articles read in the full text were examined to broaden the number of relevant articles.
Table 2. Overview of the study on the type of ketogenic diet, study design, diagnosis, ketone measurement and target levels.
Eligibility Criteria
Articles included in the review were clinical studies assessing the effect of different KD treatments in adult patients (>18 years) with glioma. Eligible criteria were original peer-reviewed articles written in English and published between 2000 and March 2022. Exclusion criteria were studies not assessing human subjects (e.g., animal studies), studies including <5 patients (e.g., case reports), and studies assessing other neurological diseases than glioma (e.g., epilepsy and Alzheimer’s disease).
Analysis of the Literature
Previous studies have focused mostly on the KDs impact on side effects and patient survival (Citation31), and to a less extent on the nutritional aspects of KDs implemented in glioma patients. Hence, this present review is descriptive and focuses primarily on the methodological approaches that were used in the different studies on KD treatments in patients with glioma. Due to differences in the definition of the KD, we categorized the dietary treatments into three main groups: the CKD, MAD, and MCT diet. The CKD was defined as diets with a 3–4:1 ketogenic ratio, MAD as a modified diet with a lower ratio of 1–2:1 or a carbohydrate intake higher than >30 g, and the MCT diet was defined as diets including >30% MCT sources of fat. Metabolic outcomes such as changes in body parameters, lipid profiles, and glucose status were recorded but not further analyzed, being beyond the scope of the present paper. Similarly, the effectiveness of tumor treatment was not part of this review and was only mentioned briefly.
Result
Characteristics of the Included Studies
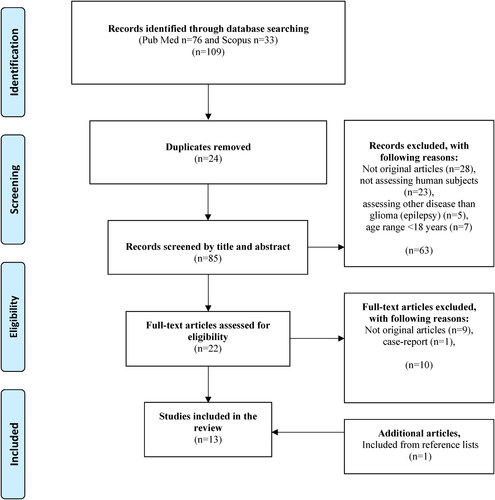
A total of 109 articles were identified by search strategy in PubMed (n = 76) and Scopus (n = 33), whereof some articles (n = 24) were duplicates (). The remaining articles (n = 85) were screened by title and abstract. Thereafter, the remaining articles (n = 22) considered relevant were read in full text. After reading full texts, articles (n = 10) were excluded that were not original articles (n = 9) and case-report (n = 1). One additional article was found through screening reference lists (Citation35). Thus, a total of 13 articles were included in the review study (Citation35–47), published between 2014–2022, and including a total number of 187 patients (men, n = 90; women, n = 55) (). Two studies did not specify the gender of the participants (n = 42) (Citation43, Citation46).
Study designs of the included articles were: clinical trial (n = 4), single-arm study (n = 4), two-arm study (n = 2), case series (n = 1), open label study (n = 1) and cohort study (n = 1) (Citation35–47). Three studies included control groups: the first study retrospectively included a control group (Citation36), the second study allocated participants to either an intervention group or control group (no randomization) (Citation43), and the third study randomized patients to a standard diet or a MAD (Citation46). One study performed a two-arm study, where they randomized participants to either a MAD or MCT diet (Citation39). One study included three cohorts: dietary intervention alone, and dietary intervention combined with low-dose or high-dose metformin (all patients received radiotherapy) (Citation37).
A total of 142 patients were diagnosed with glioblastoma (Citation35–47), 26 patients with glioma grade 3 (Citation37, Citation38, Citation40, Citation41, Citation44, Citation47), and nine patients with glioma grade 2 (Citation36, Citation40, Citation44, Citation47). One study referred to diagnosis as a “lesser grade of glioma” in two patients (Citation46). The number of participants in the studies ranged from 5 to 29 (Citation35–47). The KD treatments were initiated at different phases of targeted treatment and used in parallel with other ongoing oncological treatments. A majority studied KD treatment alongside chemotherapy (Citation35–42, Citation45, Citation47), and radiotherapy (Citation35, Citation37–40, Citation42, Citation46, Citation47). One study included patients with concurrent radiotherapy in addition to chemotherapy (Citation40). Three studies included patients with no concurrent oncologic treatment besides the KD treatment (Citation36, Citation43, Citation44). Further details of the included studies are presented in .
Identified Ketogenic Diets
Several KD regimens including different dietary characteristics were identified (). Three studies implemented a CKD as the only dietary treatment, with a ketogenic ratio of 3–4:1 (Citation35, Citation36, Citation40), seven studies applied the MAD diet (Citation38, Citation41–44, Citation46, Citation47), and two studies used the MCT diet in combination with CKD (Citation45), and MAD (Citation39). A total of three studies used more than one dietary treatment (Citation37, Citation39, Citation45). The first study applied the MAD three days before the CKD (Citation37) and was therefore classified as MAD and CKD. The second study randomized its participants to receive either MAD or MCT and was classified as MAD and MCT (Citation39). The third study was classified as CKD and MAD, as they assigned their patients (n = 9) to a CKD for 6 weeks, followed by 6 additional weeks of MAD (Citation45).
Table 3. Overview of the study regarding their type of ketogenic diet, number of participants, duration of intervention, diet restriction, follow-up, consultation with a registered dietitian, and other dietary aspects.
Fasting was induced in some patients following MAD (Citation44, Citation46). Caloric restriction of 1600 kcal/day was combined with dietary intervention in one study that applied a CKD (Citation35). Fasting was used in combination with dietary intervention in two studies applying a MAD (Citation44, Citation46). There were too few studies with caloric restriction/fasting in combination with a KD to evaluate any potential differences in diet-related outcomes or anthropometric measurements. The CKD treatments were restricted in amount of carbohydrates in two studies, with intake restricted to <20–30 g/day (Citation37, Citation40). For studies applied a MAD, with intake of carbohydrates limited to <20–60 g (Citation38, Citation42, Citation44, Citation46).
The dietary treatments duration ranged from 9 day up to over 31 mo (Citation35–47). The studies assessing CKD had a broad variation in treatment duration between 1.5 mo and 6 mo (Citation35–37, Citation40). The MAD was applied for less than a week in two studies (Citation37, Citation46), and between 1.5 mo, to 3 mo, in the remaining studies (Citation38, Citation39, Citation41–45, Citation47). The duration for the patients following the MCT diet was 3 mo (Citation39). Follow-up frequency was described in eight studies, varying from daily contact up to 6–8-week between any contact (Citation35, Citation37–39, Citation42, Citation43, Citation45, Citation47).
Adverse Events
Adverse events were measured in eleven studies (Citation35, Citation37–39, Citation41–47), whereof eight reported occurrences of adverse events () (Citation35, Citation38, Citation39, Citation41, Citation44–47). Symptoms such as constipation and diarrhea were categorized as GI symptoms. Two of four studies using a CKD treatment reported occurrence of adverse events such as GI problems (n = 9), nausea/vomiting (n = 5), fatigue/dizziness (n = 3), hypoglycemia (n = 1), hallucinations (n = 1), allergic reaction (n = 1), wound infection (n = 1) (Citation35, Citation45). The study that implemented a CKD after initiating MAD in 6 patients, found no adverse events (Citation37).
Patient who followed MAD reported GI symptoms (n = 7), nausea/vomiting (n = 8), anorexia (n = 6), hiccups (n = 1) as adverse events (Citation38, Citation41, Citation44, Citation46, Citation47). Seven studies had MAD as the only dietary intervention (Citation38, Citation41–44, Citation46, Citation47), of which five reported some adverse events (Citation38, Citation41, Citation44, Citation46, Citation47), and two had no occurrence of any adverse events (Citation42, Citation43). Some studies did not specify the number of adverse events (Citation44, Citation46), or which type of KD the patients were following (Citation39).
Other clinical measurements described as events during the study duration were hypercholesterolemia (n = 9), hypokalemia (n = 1), hypernatremia (n = 1), hypocalcemia (n = 1) hyperuricemia (n = 1) low carnitine (n = 1) (Citation39, Citation41, Citation45). Nine studies, assessing CKD, MAD or CKD and MAD, concluded that dietary treatment alongside standard care was safe and well-tolerated (Citation35, Citation38, Citation40, Citation42, Citation44–47).
Adherence to Dietary Treatment
The number of dropouts in the included studies ranged from 13 to 60% () (Citation35, Citation38–45, Citation47). Reasons for dropouts were dietary burden (e.g., too restrictive diet, difficulty in following diet) (n = 12), dietary intolerance (e.g., constipation, MCT intolerance) (n = 3), impaired quality of life (n = 3), tumor progression/recurrent glioma (n = 5), hospital admission (n = 1), family circumstances (n = 1) or recruited to another trial (n = 1) (Citation35, Citation38–45, Citation47).
One study implementing a CKD reported that three of five patients dropped out after 2 mo, but did not specify any reasons (Citation36). Another study reported that four of six patients followed the CKD for at least one month, but only one participant followed the diet for four months (Citation37). The third study had a loss follow up in three of eight patients which left the study due to disease progression (n = 2) and dietary burden (n = 1) (Citation35). One study did not report any drop-out for their patients (n = 12) commencing a CKD for 4 mo (Citation40).
Studies commencing MAD reported hospital admission (n = 1), dietary preferences (n = 6), tumor progression/recurrence (n = 3) and impaired quality of life (n = 3) and nausea (Citation38, Citation39, Citation41, Citation42, Citation47). Reasons for drop-outs in studies assessing the MAD were not mentioned in two studies (Citation44, Citation46), and one study did not specify if patients were allocated to the intervention group or the control group (Citation43). Dietary burden (n = 2), recruited to another trial (n = 1), and GI intolerance (n = 1) were reasons for drop-out in patients following MCT (Citation39).
All included studies assessed adherence to dietary treatments by ketone body production, measured in urine (Citation36, Citation38, Citation39, Citation41–44), or blood (Citation35, Citation37, Citation40, Citation41, Citation45–47) (). The interval of measuring ketone levels varied from twice daily to once weekly (Citation35–47). Ketone levels set as adherence varied between >2–5 mmol/L in urine (Citation36, Citation38, Citation39, Citation42), to >40 mmol/L (Citation43, Citation44), and between 0.5 to 3 mmol/L blood (Citation37, Citation40, Citation45, Citation47). Three studies instructed subjects to measure the blood and/or urine ketone levels at home but did not specify the level of ketone bodies set as adherence (Citation35, Citation41, Citation46). One study reported urine ketone levels set as a target but did not describe ketone levels in mmol/L (Citation36).
Evaluation of Metabolic Outcomes
Changes in anthropometric measurements were assessed in ten studies () (Citation35, Citation37, Citation40–42, Citation44, Citation46, Citation47). A significant reduction in body weight was found in most of the patients following the CKD (Citation35, Citation37, Citation40). One study reported that a majority (n = 5) lost 5–10% of their initial BMI and that some patients (n = 3) had a difference in BMI >10% after following the CKD (Citation40). Changes in anthropometric measurements were assessed in all studies assessing MAD as only dietary intervention (Citation38, Citation41–44, Citation46), whereof a trend of weight reduction was seen in four studies (Citation42, Citation44, Citation46, Citation47). Controversy results were found in two studies where no significant changes in body weight occurred in patients following a MAD (Citation38, Citation39). For patients following the MCT diet, weight changes were found insignificant (Citation39).
Lipid profile was reported unchanged in five studies (Citation37, Citation38, Citation42, Citation46), normal pre-and post-diet in one study (Citation45), and improved in two studies (Citation39, Citation43). Three of four studies evaluating the CKD assessed lipid profile (Citation35, Citation37, Citation45), whereof no study found a significant before and after the dietary intervention. Similarly, the result was found in patients following MAD where most of the studies did not find any significant difference in lipid profile (Citation37, Citation38, Citation41, Citation42, Citation45, Citation46). Reduction of LDL cholesterol was found in two studies (Citation43, Citation44). Glucose levels were assessed in four studies (Citation35, Citation37, Citation41, Citation44, Citation46). No significant change in fasting glycemia or HbA1c was found in patients following the CKD (Citation37). A significant reduction of glucose levels was seen in patients commencing MAD combined with intermittent fasting (Citation46). Nevertheless, two other studies assessing MAD found no changes in blood glucose levels (Citation41, Citation44).
Clinical Outcome Measures
Overall survival and progression-free survival were the most frequently assessed as clinical outcomes and reported in seven (Citation35, Citation39, Citation41, Citation42, Citation45–47), and four studies respectively (Citation39, Citation41, Citation42, Citation46) ().
Table 4. Overview of the study results on adverse events, adherence, and metabolic outcomes.
Table 5. Overview of the study results on clinical outcomes, diet tolerance, and quality of life.
Overall survival was reported with a span of 1.3–38.7 mo, for patients diagnosed with glioblastoma (Citation35, Citation39, Citation42, Citation45), assessing the effects of CKD (Citation35), MAD and MCT (Citation39), MAD (Citation42), or CKD and MAD (Citation45). Overall survival was reported in a range of 4–56 mo, in patients with different glioma malignities following a CKD (Citation40). One study that applied a MAD in patients with either recurrent glioblastoma or lower grade brain tumor found no differences in overall survival or progression-free survival (Citation46). On the other hand, another study also applying MAD as a treatment intervention found that median progression-free survival was 10 mo, for newly diagnosed patients and 4 mo, for patients with recurrent disease, with a median overall survival of 21 mo, and 8 mo, respectively (Citation41). A third study reported a trend toward longer progression-free survival in the patient group (6 weeks) with stable ketosis (n = 8) compared to other patients (3 weeks, n = 5), in patients diagnosed with glioblastoma attaining a MAD (Citation42).
Seizure control was only measured as a clinical outcome in two studies (Citation40, Citation46). One study found that most participants (n = 9) who commenced a CKD for 4 mo, did not experience any seizures during dietary treatment. Seizures were increased in severity for one patient and improved in two patients (Citation40). One study assessing patients following a MAD reported that most of the adverse events reported were epileptic seizures but did not report specific numbers (Citation46).
Diet Tolerance
Various aspects of diet tolerance, such as malnutrition, hunger, quality of life, appetite, nausea/vomiting were assessed in nine studies () (Citation35–40, Citation42, Citation45, Citation46). One study evaluating the CKD for three months reported that appetite decreased in five of twelve patients, improved in four, and was not reported in three (Citation40). Complaints regarding diet tolerance of CKD were reported in other studies, including one patient who stopped the diet early because of diet restrictiveness (Citation35). Also, patients with partners found the diet more challenging than expected (Citation45). Patients commencing a CKD reported increased hunger (Citation35) and similar results were seen for patients following the MAD (Citation46).
Quality of Life
Quality of life (QoL) was assessed in three studies assessing the MAD (Citation42), MAD and MCT (Citation39), and CKD (Citation45) (). Patients with recurrent glioblastoma (n = 20) following the MAD reported that 15% experienced that restricted carbohydrate intake negatively affected their QoL (Citation42). Contradictory, patients (n = 6) in another study also following a MAD reported that they would recommend the diet to other patients, especially after surgery and before radiotherapy and that they would consider the possibility to follow the diet for longer than 12 weeks in a clinical trial (Citation39). In a third study, the QoL was measured at three time points (baseline, after 6 weeks, and at the end of the study) in participants following a CKD for 6 weeks followed by a MAD (Citation45). The values of QoL were scored 0–100 and compared to a Dutch cancer survival population. The research group found normal values in QoL when compared to the normative data for a majority of the study participants at the start and at the end of the study (Citation45).
Discussion
Although it has been known for almost a century that KD may have a starving effect on the glioma cells, KD treatment is not used as a standard treatment in neuro-oncology. Prior to introduction, we need to know how to use this diet safely and most effectively in patients with gliomas. Reviewing the literature on this subject, we identified several differences in methodological approaches of KD treatments.
Dietary Characteristics of Included Studies
The KD treatments varied largely in dietary characteristics, as ketogenic ratios were described as 1:1 to 4:1, and restriction of carbohydrates ranged between 20–60 g. In addition, we identified studies implementing fasting or caloric energy reduction alongside the dietary treatment and/or boosting the MAD with MCT oil to induce ketosis. This broad definition of a KD and lack of standard methods make it difficult to study the effect of the KD in glioma. This variation of KDs used in cancer patients has been identified in previous research (Citation17, Citation31), and future studies should acknowledge these methodological issues also in glioma patients.
Feasibility of the Dietary Treatments
Most of the included studies reported several adverse events in patients following a KD, whereof GI problems were the most reported one. Adverse events related to GI symptoms, such as constipation, gastrointestinal disturbance, and vomiting, have been reported as common side effects of a KD (Citation48). In this review, we found that a higher proportion of GI symptoms were reported in patients following a CKD compared to the MAD, which is similar to previous research which has stated the MAD as a treatment with fewer adverse effects compared to the CKD (Citation16). However, the limited number of study participants makes it difficult to draw any further conclusions. It should also be noted that most participants received concurrent cancer treatment alongside diet therapy, and adverse events possibly related to dietary treatment should therefore be analyzed with caution.
Adherence to Ketogenic Diets
The ketosis levels set as a threshold for adherence varied largely between the different studies. This implies that a patient in one study can be considered compliant, while another patient with the same ketone levels in another study may be excluded due to noncompliance. Currently, there are no recommendations for optimal ketone levels in adults with epilepsy (Citation49). Pediatric ketogenic services often recommend a blood BHB level of 4–6 mmol/L, although some patients can achieve optimal seizure control with lower ketone levels (Citation49). Due to these discrepancies, it is problematic to compare the results and effects of the KD between such studies.
As the KD treatments differ in imposing dietary burden, it is important to follow up on the patient’s individual needs and preferences (Citation50), and weekly contact has been considered optimal for maintenance (Citation51). In this review, patients were followed up daily up to 6–8 weeks intervals, whereof only two studies reported weekly contact with the patients. Similar results have been presented in a previous systematic review, which concluded that diet delivery of the KD lacked follow-up (Citation17). These different time intervals may have affected patients’ ability to achieve ketosis. Hence, it would be desirable to consider such aspects in future trial designs to avoid non-adherence.
We identified a variation in access to additional dietary support and consultations with a dietitian in the studies. As parameters of dietary tolerance were scarcely reported, and assessed in only a few studies, no strong recommendations for future trial design can be made based on published studies so far. Diet restrictiveness, complexity, and restrictions of social and culinary aspects have been described as possible factors causing the high dropout rate of the KD (Citation52). Henceforth, diet tolerance should be further investigated while implementing a KD to increase the patient satiety and improve adherence.
Strengths and Limitations
We found mixed results regarding adverse events, changes in anthropometric measurements, and survival and we did not observe any clear trend in correlations between the dietary treatments with glucose levels or lipid profile. This might be explained by the limited number of papers that were scouted, although we used a broad approach to search for relevant literature. The small number of studies available is an obvious limitation of this review but is likely to reflect the current situation since a broad approach was used to identify eligible articles. This range of methodological characteristics suggests a striking lack of standard methods to measure the efficacy of KD in this patient group. Furthermore, studying adverse effects in this type of study is challenging, due to the complexity to separate adverse events caused by standard of care from adverse events caused by dietary treatment. Therefore, we suggest that consensus is sought to at least bring forward recommendations of KD on the presumed need for metabolic changes to occur and, importantly, to establish the minimum level of ketosis acceptable in clinical trials.
Conclusion
There is a heterogenicity in methodological approaches of KD treatments in glioma, and in estimating the effect measured in ketone levels. Due to these discrepancies in dietary characteristics, it is difficult to compare the results and effects of KD treatment between the studies. We conclude that there is an unmet need for standardized KD regimes and a consensus on methodological implementation for studies to be comparable. Therefore, we suggest that consensus is sought to at least bring forward recommendations of KD on the presumed need for metabolic changes to occur, and importantly also to establish the minimum level of ketosis acceptable in clinical trials but also to investigate to what extent KD can be optimized to increase adherence, to secure optimal nutrient status and patient satisfaction during treatment.
Author’s Contribution
Designed by A.G and A.S.J. A.G performed the literature search, and analysis of articles included and prepared the manuscript in consultation with F.A, A.S, and A.S.J. All authors were involved in writing the manuscript, the submission process, and approved the final manuscript.
Supplemental Material
Download MS Word (14.6 KB)Disclosure Statement
The authors declare no conflict of interest.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Gusyatiner O, Hegi ME. Glioma epigenetics: from subclassification to novel treatment options. Semin Cancer Biol. 2018;51:50–8. doi:10.1016/j.semcancer.2017.11.010
- Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, Pekmezci M, Schwartzbaum JA, Turner MC, Walsh KM, et al. The epidemiology of glioma in adults: a "state of the science" review. Neuro Oncol. 2014;16(7):896–913. doi:10.1093/neuonc/nou087
- Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23(8):1231–51. doi:10.1093/neuonc/noab106
- Ostrom QT, Cote DJ, Ascha M, Kruchko C, Barnholtz-Sloan JS. Adult glioma incidence and survival by race or ethnicity in the United States from 2000 to 2014. JAMA Oncol. 2018;4(9):1254–62. doi:10.1001/jamaoncol.2018.1789
- Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven L, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170–86. doi:10.1038/s41571-020-00447-z
- Wang TJC, Mehta MP. Low-grade glioma radiotherapy treatment and trials. Neurosurg Clin N Am. 2019;30(1):111–8. doi:10.1016/j.nec.2018.08.008
- Klement RJ, Kämmerer U. Is there a role for carbohydrate restriction in the treatment and prevention of cancer? Nutr Metab (Lond). 2011;8(1):75. doi:10.1186/1743-7075-8-75
- Strowd RE, Cervenka MC, Henry BJ, Kossoff EH, Hartman AL, Blakeley JO. Glycemic modulation in neuro-oncology: experience and future directions using a modified Atkins diet for high-grade brain tumors. Neurooncol Pract. 2015;2(3):127–36. doi:10.1093/nop/npv010
- Branco AF, Ferreira A, Simões RF, Magalhães-Novais S, Zehowski C, Cope E, Silva AM, Pereira D, Sardão VA, Cunha-Oliveira T, et al. Ketogenic diets: from cancer to mitochondrial diseases and beyond. Eur J Clin Invest. 2016;46(3):285–98. doi:10.1111/eci.12591
- Verrotti A, Iapadre G, Pisano S, Coppola G. Ketogenic diet and childhood neurological disorders other than epilepsy: an overview. Expert Rev Neurother. 2017;17(5):461–73. doi:10.1080/14737175.2017.1260004
- Paoli A, Bianco A, Damiani E, Bosco G. Ketogenic diet in neuromuscular and neurodegenerative diseases. Biomed Res Int. 2014;2014:474296. doi:10.1155/2014/474296
- Levy RG, Cooper PN, Giri P. Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst Rev. 2012;(3):CD001903. doi:10.1002/14651858.CD001903.pub2
- Ułamek-Kozioł M, Czuczwar SJ, Januszewski S, Pluta R. Ketogenic diet and epilepsy. Nutrients. 2019;11(10):2510. doi:10.3390/nu11102510
- Lee PR, Kossoff EH. Dietary treatments for epilepsy: management guidelines for the general practitioner. Epilepsy Behav. 2011;21(2):115–21. doi:10.1016/j.yebeh.2011.03.008
- Payne NE, Cross JH, Sander JW, Sisodiya SM. The ketogenic and related diets in adolescents and adults – a review. Epilepsia. 2011;52(11):1941–8. doi:10.1111/j.1528-1167.2011.03287.x
- Martin-McGill KJ, Bresnahan R, Levy RG, Cooper PN. Ketogenic diets for drug‐resistant epilepsy. Cochrane Database Syst Rev. 2020;2020(6). Art. No.: CD001903. doi:10.1002/14651858.CD001903.pub5 (Accessed 05 August 2022).
- Sremanakova J, Sowerbutts AM, Burden S. A systematic review of the use of ketogenic diets in adult patients with cancer. J Hum Nutr Diet. 2018;31(6):793–802. doi:10.1111/jhn.12587
- Schwartz KA, Noel M, Nikolai M, Chang HT. Investigating the ketogenic diet as treatment for primary aggressive brain cancer: challenges and lessons learned. Front Nutr. 2018;5:11. doi:10.3389/fnut.2018.00011
- Kossoff EH, Krauss GL, McGrogan JR, Freeman JM. Efficacy of the Atkins diet as therapy for intractable epilepsy. Neurology. 2003;61(12):1789–91. doi:10.1212/01.wnl.0000098889.35155.72
- Kossoff EH, Dorward JL. The modified Atkins diet. Epilepsia. 2008;49(Suppl 8):37–41. doi:10.1111/j.1528-1167.2008.01831.x
- Kang HC, Chung DE, Kim DW, Kim HD. Early- and late-onset complications of the ketogenic diet for intractable epilepsy. Epilepsia. 2004;45(9):1116–23. doi:10.1111/j.0013-9580.2004.10004.x
- Liu YM, Wang HS. Medium-chain triglyceride ketogenic diet, an effective treatment for drug-resistant epilepsy and a comparison with other ketogenic diets. Biomed J. 2013;36(1):9–15. doi:10.4103/2319-4170.107154
- Kverneland M. Modified ketogenic (Atkins) diet as a treatment option for adults with drug-resistant epilepsy [dissertation on the Internet]. Oslo: University of Oslo; 2019. [cited 2021 Apr 27]. Available from: http://urn.nb.no/URN:NBN:no-76817.
- Weber DD, Aminzadeh-Gohari S, Tulipan J, Catalano L, Feichtinger RG, Kofler B. Ketogenic diet in the treatment of cancer - where do we stand? Mol Metab. 2020;33:102–21.
- Pfeifer HH, Thiele EA. Low-glycemic-index treatment: a liberalized ketogenic diet for treatment of intractable epilepsy. Neurology. 2005;65(11):1810–2. doi:10.1212/01.wnl.0000187071.24292.9e
- Abdelwahab MG, Fenton KE, Preul MC, Rho JM, Lynch A, Stafford P, Scheck AC. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS One. 2012;7(5):e36197. doi:10.1371/journal.pone.0036197
- Seyfried TN, Flores R, Poff AM, D’Agostino DP, Mukherjee P. Metabolic therapy: a new paradigm for managing malignant brain cancer. Cancer Lett. 2015;356(2 Pt A):289–300. doi:10.1016/j.canlet.2014.07.015
- Thomas JG, Veznedaroglu E. Ketogenic diet for malignant gliomas: a review. Curr Nutr Rep. 2020;9(3):258–63. doi:10.1007/s13668-020-00332-2
- Noorlag L, De Vos FY, Kok A, Broekman MLD, Seute T, Robe PA, Snijders TJ. Treatment of malignant gliomas with ketogenic or caloric restricted diets: a systematic review of preclinical and early clinical studies. Clin Nutr. 2019;38(5):1986–94. doi:10.1016/j.clnu.2018.10.024
- Varshneya K, Carico C, Ortega A, Patil CG. The efficacy of ketogenic diet and associated hypoglycemia as an adjuvant therapy for high-grade gliomas: a review of the literature. Cureus. 2015;7(2):e251.
- Sargaço B, Oliveira PA, Antunes ML, Moreira AC. Effects of the ketogenic diet in the treatment of gliomas: a systematic review. Nutrients. 2022;14(5):1007. doi:10.3390/nu14051007
- Chung HY, Park YK. Rationale, feasibility and acceptability of ketogenic diet for cancer treatment. J Cancer Prev. 2017;22(3):127–34. doi:10.15430/JCP.2017.22.3.127
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. doi:10.1016/j.jclinepi.2009.06.005
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:https://doi.org/10.1136/bmj.n71.
- Klein P, Tyrlikova I, Zuccoli G, Tyrlik A, Maroon JC. Treatment of glioblastoma multiforme with "classic" 4:1 ketogenic diet total meal replacement. Cancer Metab. 2020;8(1):24. doi:10.1186/s40170-020-00230-9
- Artzi M, Liberman G, Vaisman N, Bokstein F, Vitinshtein F, Aizenstein O, Ben Bashat D. Changes in cerebral metabolism during ketogenic diet in patients with primary brain tumors: (1)H-MRS study. J Neurooncol. 2017;132(2):267–75. doi:10.1007/s11060-016-2364-x
- Foppiani A, De Amicis R, Lessa C, Leone A, Ravella S, Ciusani E, Silvani A, Zuccoli G, Battezzati A, Lamperti E, et al. Isocaloric ketogenic diet in adults with high-grade gliomas: a prospective metabolic study. Nutr Cancer. 2021;73(6):1004–14. doi:10.1080/01635581.2020.1779759
- Martin-McGill KJ, Marson AG, Tudur Smith C, Jenkinson MD. The modified ketogenic diet in adults with glioblastoma: an evaluation of feasibility and deliverability within the National Health Service. Nutr Cancer. 2018;70(4):643–9. doi:10.1080/01635581.2018.1460677
- Martin-McGill KJ, Marson AG, Tudur Smith C, Young B, Mills SJ, Cherry MG, Jenkinson MD. Ketogenic diets as an adjuvant therapy for glioblastoma (KEATING): a randomized, mixed methods, feasibility study. J Neurooncol. 2020;147(1):213–27. doi:10.1007/s11060-020-03417-8
- Panhans CM, Gresham G, Amaral LJ, Hu J. Exploring the feasibility and effects of a ketogenic diet in patients with CNS malignancies: a retrospective case series. Front Neurosci. 2020;14:390. doi:10.3389/fnins.2020.00390
- Porper K, Shpatz Y, Plotkin L, Pechthold RG, Talianski A, Champ CE, Furman O, Shimoni-Sebag A, Symon Z, Amit U, et al. A phase I clinical trial of dose-escalated metabolic therapy combined with concomitant radiation therapy in high-grade glioma. J Neurooncol. 2021;153(3):487–96. doi:10.1007/s11060-021-03786-8
- Rieger J, Bähr O, Maurer GD, Hattingen E, Franz K, Brucker D, Walenta S, Kämmerer U, Coy JF, Weller M, et al. ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol. 2014;44(6):1843–52. doi:10.3892/ijo.2014.2382
- Santos JG, Da Cruz WMS, Schönthal AH, Salazar MD, Fontes CAP, Quirico-Santos T, Da Fonseca CO. Efficacy of a ketogenic diet with concomitant intranasal perillyl alcohol as a novel strategy for the therapy of recurrent glioblastoma. Oncol Lett. 2018;15(1):1263–70.
- Schreck KC, Hsu F-C, Berrington A, Henry-Barron B, Vizthum D, Blair L, Kossoff EH, Easter L, Whitlow CT, Barker PB, et al. Feasibility and biological activity of a ketogenic/intermittent-fasting diet in patients with glioma. Neurology. 2021;97(9):e953–e63. doi:10.1212/WNL.0000000000012386
- van der Louw EJTM, Reddingius RE, Olieman JF, Neuteboom RF, Catsman-Berrevoets CE. Ketogenic diet treatment in recurrent diffuse intrinsic pontine glioma in children: a safety and feasibility study. Pediatr Blood Cancer. 2019;66(3):e27561. doi: 10.1002/pbc.27561
- Voss M, Wagner M, von Mettenheim N, Harter PN, Wenger KJ, Franz K, Bojunga J, Vetter M, Gerlach R, Glatzel M, et al. ERGO2: a prospective, randomized trial of calorie-restricted ketogenic diet and fasting in addition to reirradiation for malignant glioma. Int J Radiat Oncol Biol Phys. 2020;108(4):987–95. doi:10.1016/j.ijrobp.2020.06.021
- Woodhouse C, Ward T, Gaskill-Shipley M, Chaudhary R. Feasibility of a modified atkins diet in glioma patients during radiation and its effect on radiation sensitization. Curr Oncol. 2019;26(4):e433–e438.
- Wells J, Swaminathan A, Paseka J, Hanson C. Efficacy and safety of a ketogenic diet in children and adolescents with refractory epilepsy - a review. Nutrients. 2020;12(6):1809. doi:10.3390/nu12061809
- Schoeler NE, Cross JH. Ketogenic dietary therapies in adults with epilepsy: a practical guide. Pract Neurol. 2016;16(3):208–14. doi:10.1136/practneurol-2015-001288
- Zupec-Kania B, Neal E, Schultz R, Roan ME, Turner Z, Welborn M. An update on diets in clinical practice. J Child Neurol. 2013;28(8):1015–26. doi:10.1177/0883073813487597
- Schwartz K, Chang HT, Nikolai M, Pernicone J, Rhee S, Olson K, Kurniali PC, Hord NG, Noel M. Treatment of glioma patients with ketogenic diets: report of two cases treated with an IRB-approved energy-restricted ketogenic diet protocol and review of the literature. Cancer Metab. 2015;3:3. doi:10.1186/s40170-015-0129-1
- Clemente Fuentes RW, Broszko CM, Pietralcyzk ES, Nashelsky J. Efficacy of a low-carbohydrate or ketogenic diet in preventing patient morbidity and mortality. Can Fam Physician. 2020;66(4):262–3.