Abstract
The effects of fish oil for improving mental health have been reported. The present study was undertaken to compare the effects of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) on anxiety-like behavior using a rat model. Experimental diets enriched in EPA or DHA as glycerides were prepared. Rats were exposed to social isolation stress and fed the experimental diet for 14 days. The results of behavioral tests revealed that rats fed the EPA-enriched diet exhibited less anxiety-like behavior than rats fed the control or DHA-enriched diets. Furthermore, EPA suppressed anxiety-like behavior only in socially isolated rats. The increase in EPA contents in the brain phospholipid fraction by feeding EPA-enriched diet was more significant than that of DHA by feeding DHA-enriched diet. These results suggest that dietary EPA is more anxiolytic than DHA in rats exposed to social isolation stress and is effective in increasing EPA content in brain membranes.
This study revealed that EPA has a stronger inhibitory effect on anxiety-like behaviors than DHA in rats under stress.
Since reports indicated that the intake of fish oil reduces the risk of depression in humans [Citation1,2], the influence of n-3 polyunsaturated fatty acids (PUFA) contained in fish oil on brain function has attracted attention. Many studies have reported that n-3 PUFA are effective for the prevention and mitigation of mental illness [Citation3]; however, when summarizing various research examples, their effectiveness remains unclear [Citation4–6]. Because depression and anxiety share many similar characteristics [Citation7], the possibility that n-3 PUFA have anxiolytic effects has also been investigated. It has been shown that intracellular n-3 PUFA levels in patients experiencing anxiety and depression are low and that anxiety can be alleviated by the addition of n-3 type PUFA [Citation8,9].
Research investigating n-3 PUFA and anxiety in laboratory animals is ongoing. It has been reported that deficiency in n-3 PUFA increases anxiety and fear in rats caused by separation from mothers [Citation10]. Although it has been reported that ingestion of n-3 PUFA affects anxiety and depression-like behaviors in rodents [Citation11,12], consistent results have not necessarily been obtained [Citation13]. Difference in the status of animals used for experiments is considered one of the causes of inconsistent results, and the effect of n-3 PUFA tends to be especially robust when the animals used in experiments are in a state of stress [Citation14,15].
While the influence of fish oil and n-3 PUFA has been intensively studied, it has not been clarified which n-3 PUFA affect anxiety-like behavior. Among n-3 PUFA, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), both of which can be synthesized from α-linolenic acid, are considered to exert strong actions on brain functions. Concentrations of EPA and DHA in the body are greatly affected by dietary intake because the synthesis activity of these fatty acids from α-linolenic acid is not especially high [Citation16]. Many experiments investigating the effect of n-3 PUFA on emotions, such as anxiety, were performed with safflower oil or peanut oil, which contains little n-3 PUFA, combined with fish oil or rapeseed oil rich in n-3 PUFA or α-linolenic acid, respectively [Citation17–20]. Mizunoya et al. compared the effect of fish oil and lard on anxiety and reported that anxiety-like behavior in the elevated plus maze (EPM) test was reduced in the fish oil group [Citation21]. For the purpose of examining the influence of individual fatty acids, diets supplemented with ethylated n-3 fatty acid [Citation22,23] and supplemented with each n-3 fatty acid were used [Citation24]. The results of experiments in which rats were administered α-linolenic acid, EPA, or DHA demonstrated no change in anxiety-like behavior [Citation24]. However, one study reported that intake of ethylated EPA suppressed anxiety [Citation22].
In the present study, we aimed to compare the anxiolytic effect of EPA and DHA in rats using oils enriched with EPA or DHA as glycerides. Because the influence of n-3 PUFA on anxiety may be strongly influenced by stress, we studied rats that were subjected to social isolation stress by individually housing the animals.
Materials and methods
Materials
EPA-40 and DHA-46 were provided by Tama Biochemical Co, Ltd (Tokyo, Japan). These oils are enriched with EPA (40% w/w) or DHA (46% w/w), respectively, mostly in diglyceride and triglyceride forms.
Animal experiments
Three-week-old male Wistar rats were purchased (Japan Laboratory Animals, Inc., Tokyo, Japan) and housed at 22–24°C under a 12 h light/dark cycle (06:00–18:00). The animals were fed a commercial pellet diet (certified diet MF; Oriental Yeast, Tokyo, Japan) ad libitum for the first 3 days to acclimate to the new environment before each experiment. Rats were housed individually in stainless steel cages (150 × 240 × 110 mm) and were allowed ad libitum access to the diet and water throughout the experiment. The EPM test was performed on days 15 and 16 after commencement of the experimental diets, and the open-field (OF) test was performed on days 17 and 18. The rats were euthanized on day 19 under anesthesia with sodium pentobarbital (1 mg/kg, intraperitoneal, Somnopentyl, Kyoritsu Pharmaceutical Co. Ltd, Tokyo, Japan); plasma and whole brains were obtained and stored at −80°C until use. All animal handling and experimental procedures were approved by the Meiji University Institutional Animal Care and Use Committee (Tokyo, Japan).
Animal experiment 1
Eighteen rats were divided into 3 groups (n = 6 each) and fed a control (CON), EPA-enriched (EPA), or DHA-enriched (DHA) diet for 19 days. The compositions of the experimental diets are presented in Table . The animals were housed individually in polypropylene cages (170 × 330 × 200 mm).
Table 1. Composition of the diets (g/100 g of diet).
Animal experiment 2
Twenty-four rats were divided into 2 groups (n = 12 each) and fed CON or EPA for 19 days. Six rats in each dietary group were housed individually in stainless steel cages (150 × 240 × 110 mm); the other 6 rats were housed in groups of 3 rats/cage in stainless steel cages (200 × 300 × 110 mm). Stainless steel cages with low height (110 mm) were used in order to stress the rats.
Fatty acid composition
Total lipid was extracted from 50 mg of the cerebral cortex of the rat brains according to the method described by Bligh and Dyer [Citation25] and dissolved in 1 mL of hexane. The lipid was saponified with an equal volume of 4 N KOH in methanol for 30 min at 60°C, centrifuged at 355 × g for 2 min, and the upper layer was extracted. The lower layer was re-extracted with 1 mL hexane and 200 μL of 4 N HCl in methanol, followed by extraction with 1 mL hexane. The extracts were combined and dried with N2 gas. The dried samples were methylated by incubating with 10% H2SO4 in methanol (v/v) for 60 min at 80°C. After 1 mL hexane and 1 mL water were added and centrifuged at 355 ×g for 2 min twice, the combined upper layers were analyzed using a gas chromatograph (GC-8A, SHIMADZU) equipped with an FID detector using a 10% SILAR 10C column (3 mm × 2 m). The column temperature was set at 250°C, and the injector at 210°C. N2 was used as the carrier gas, with a flow rate of 25 mL/min. For the analysis of phospholipid fraction, extracted total lipids were dissolved in chloroform, spotted on a thin-layer chromatography plate (Silica gel 60, Merck Millipore, USA) and separated using hexane:ether (8:2 v/v) as eluents. After the plates were developed in 2% fluorescein in ethanol under ultraviolet light, the spots at RF = 0 were scraped as phospholipid fraction and applied to methylation and gas chromatograph analysis as described above. Regarding analysis of the corn oil, EPA-40 and DHA-46, an aliquot of each sample was directly methylated and analyzed as described above.
EPM test
An X-shape EPM consisting of two open-arms (50 × 10 cm) and two closed arms (50 × 10 × 40 cm) and elevated 50 cm from the floor was used. The rats were placed individually in the center of the maze facing a closed arm and were allowed to freely explore for 15 min. Behavior was recorded using a charge-coupled device camera and automatically analyzed using a computer with software (TimeEP1 for EPM, O’hara & Co., Ltd., Tokyo Japan). The illumination level was lower than 0.5 lux during the experiment. The following behaviors were analyzed visually: activity on the open-arms; head dipping (downward movement of the head toward the edge of the open-arms), stretch-out posture (stretching the head forward without stepping forward), and locomotion. Open-arm activity and head dipping are behaviors observed in less-anxious rats, while stretch-out posture is generally observed in anxious rats.
OF test
The OF consisted of a square area (80 × 80 × 45 cm for animal experiment 1; 45 × 45 × 45 cm for animal experiment 2) made from a matte-finished black acryl panel. The squares in the center of the field (60 × 60 cm for animal experiment 1; 22.5 × 22.5 cm for animal experiment 2) were defined as the center area. The rats were placed individually in the center of the field and were allowed to freely explore for 15 min. The room was brightly illuminated with incandescent lightning during the experiment, and behavior was video recorded (HDR-CX420, Sony, Tokyo, Japan). The following behaviors were analyzed visually: activity in the center area, supported rearing (standing on the hind legs with hands on the wall), unsupported rearing (standing on hind legs), grooming, and locomotion. Rearing and grooming are behaviors observed in less-anxious rats.
Statistical analysis
The Student’s t test or Welch’s t test, accounting for equal or unequal variances, respectively, was applied to compare the values between two experimental groups. Two-way analysis of variance (ANOVA) was performed to evaluate the significant effects of diet and housing and the interaction of diet × housing. Post hoc comparisons were performed using Turkey’s test when significant interaction of diet × housing was observed using two-way ANOVA. p < 0.05 was considered to be statistically significant. All statistical analyses were performed using Statistics 2008 (Social Survey Research Information Co., Ltd., Tokyo, Japan) for Excel (Microsoft Corporation, Redmond, WA, USA).
Results
Fatty acid composition of the diet
According to the results of the fatty acid analysis of EPA-40 and DHA-46, the fatty acid composition of each diet was calculated and presented in Table . Compared with the CON diet, the EPA- and DHA-enriched diets contained more PUFA. EPA content in the EPA-enriched diet and DHA content in DHA-enriched diet were comparable.
Behavioral tests of rats fed EPA or DHA
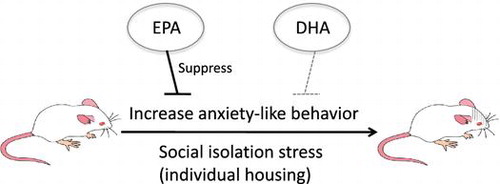
During the 19-day experimental period, food intake and body weight of the rats were not significantly different among the three dietary groups (data now shown). In the EPM test, rats fed the EPA-enriched diet exhibited significantly higher open-arm activity and lower levels of stretch-out posture compared with those in the CON- or DHA-fed groups, suggesting that EPA intake decreased anxiety-like behavior (Figure ). In the OF test, rats in the EPA-fed group devoted more time to the center area and exhibited more unsupported rearing than rats in the other groups (Figure ). The results of both tests demonstrated that EPA intake decreased anxiety-like behavior, while DHA intake did not have any effect. Because there was no change in locomotor activity, it was presumed that DHA or EPA intake did not affect motor activity.
Figure 1. Performance in the elevated plus maze test. Open-arm time (A), open-arm frequency (B), head-dipping time (C), head-dipping frequency (D), stretch-out time (E), stretch-out frequency (F), locomotion time (G), and locomotion frequency (H) in rats fed control (CON), eicosapentaenoic acid-enriched (EPA), or docosahexaenoic acid-enriched (DHA) diets.
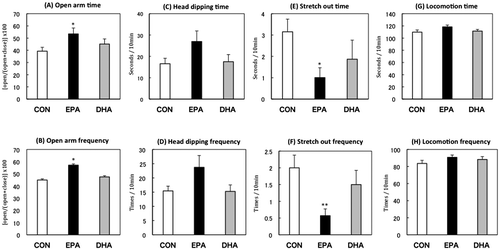
Figure 2. Performance in the open-field test. Center area time (A), center area frequency (B), supported rearing time (C), supported rearing frequency (D), unsupported rearing time (E), unsupported rearing frequency (F), grooming time (G), grooming frequency (H), locomotion time (I), locomotion frequency (J) of the rats fed control (CON), eicosapentaenoic acid-enriched (EPA), or docosahexaenoic acid-enriched (DHA) diets.
EPA and DHA contents of brain phospholipid in rats fed EPA or DHA
EPA was undetectable (i.e. < 0.01%) in the cerebral cortex of the rats fed the CON diet, while it was detectable in the brains of the EPA- and DHA-fed groups. EPA and DHA content was significantly higher in EPA- and DHA-fed groups compared with the CON group (Table ). The fold increase in EPA content was larger than that of DHA content.
Table 2. EPA and DHA contents in the phospholipid fraction of the cerebral cortex (%).
Behavior tests of rats fed EPA under stressed or non-stressed conditions
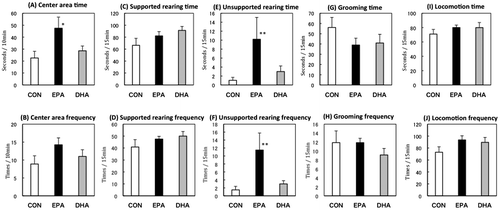
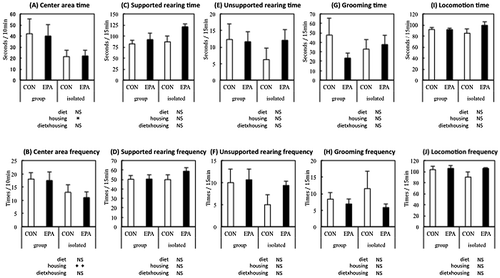
During the 19-day experimental period, food intake and body weight of the rats were not affected by housing conditions and/or diet (data not shown). In the EPM test, isolated housing decreased open-arm activity and head dipping and increased stretch-out posture, which suggested that social isolation increased anxiety-like behavior. The anxiogenic effect of social isolation was impaired in rats fed EPA. In the OF test, isolated housing influenced activity in the center area, which was not affected by the EPA diet (Figures and ).
Figure 3. Performance in the elevated plus maze test. Open-arm time (A), open-arm frequency (B), head-dipping time (C), head-dipping frequency (D), stretch-out time (E), stretch-out frequency (F), locomotion time (G), locomotion frequency (H) of the rats fed control (CON) or eicosapentaenoic acid-enriched (EPA) diets, and group-housed (group) or individually housed (i.e. isolated).
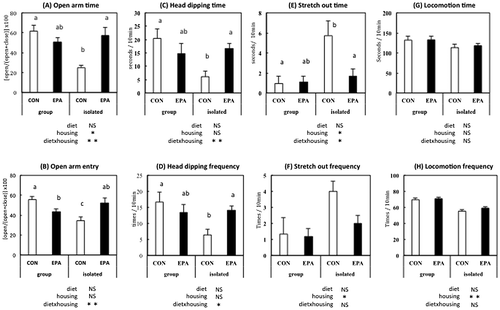
Figure 4. Performance in the open-field test. Center area time (A), center area frequency (B), supported rearing time (C), supported rearing frequency (D), unsupported rearing time (E), unsupported rearing frequency (F), grooming time (G), grooming frequency (H), locomotion time (I), and locomotion frequency (J) of the rats fed control (CON) or eicosapentaenoic acid-enriched diet (EPA) and group-housed (group) or individually housed (i.e. isolated).
Fatty acid composition in brain total lipid and phospholipid fraction of rats fed EPA
An EPA diet increased the relative amount of n-3 PUFA and decreased n-6 PUFA in total lipid fraction and phospholipid fraction in the cerebral cortex. The fold increase in EPA by feeding EPA-enriched diet was larger and more significant in the phospholipid fraction than in the total lipid fraction (Table ).
Table 3. Fatty acid composition of the cerebral cortex (%).
Discussion
Although the effects of n-3 PUFA on mental health are widely studied using natural oils, such as fish oil, studies investigating the effects of a particular fatty acid have been limited [Citation17–20]. From the results of the fatty acid measurement of the oils, we confirmed that the experimental diets used in this study contained high concentrations of EPA and DHA independently of one another. In particular, the EPA-40 and DHA-46 used in this study contained EPA and DHA in diglyceride or triglyceride form, which made it possible to investigate the effect of EPA and DHA in conditions approximating those present in natural oils.
Although the amounts of EPA and DHA intake were comparable in this study, open-arm activity in the EPM test and center area activity in the OF test were reduced significantly only in the EPA-fed group. Because these activities were the primary indicators in the behavioral tests, the results clearly demonstrated that EPA had a stronger effect in reducing anxiety-like behavior than DHA. In addition, the decrease in stretch-out posture in the EPM test and the increase in unsupported rearing in the OF test in the EPA-fed rats also reflected the stronger anxiolytic activity of EPA. In a previous report comparing the impact of EPA and DHA on anxiety, neither fatty acid affected anxiety-like behavior in rats [Citation24]. This discrepancy occurred probably because the study by Ross et al. [Citation24] differed from our study in that EPA and DHA were added as fatty acids and the test rats were not stressed. Stressing the animals by housing them individually in our experiments presumably enhanced the effect of EPA. With regard to the effect of EPA, it has been reported that the antidepressant effect of EPA was stronger than α-linolenic acid and DHA [Citation6], and intake of ethyl-EPA suppressed anxiety [Citation22]. The diet used in the study by Song et al. [Citation22] contained 20% EPA and 8% DHA, which was similar to the EPA-enriched diet in the present study. Considering these previous reports, the present study is one of the first to clearly demonstrate the more significant effect of EPA on anxiety compared with DHA.
Next, we examined whether the anxiolytic effect of EPA is peculiar to animals that are potentially more anxious due to social isolation stress exerted by individual housing. The results of the EPM test demonstrated that anxiety-like behavior was increased in individually housed rats, similar to previous reports [Citation26]. The anxiolytic effect of EPA was observed only in socially isolated rats and did not affect anxiety-like behavior in group-housed rats. Therefore, it was revealed that EPA ingestion particularly suppressed increased anxiety due to social isolation stress. In the OF test, there was no difference in behavior index among the groups, which was probably because the size of the field used in the experiment was too small to properly detect changes in anxiety-like behavior. The fact that the effect of EPA was specific to the stressed rats is consistent with a report that the influence of n-3 PUFA deficiency was observed only in individually housed rats compared with group-housed rats [Citation15].
To investigate whether the ingested fatty acids reached the brain, which is important for emotional control, the EPA and DHA content in the phospholipid fraction of the brain was measured. The contents of both EPA and DHA in the brain phospholipid fraction were increased by the consumption of a fatty acid-enriched diet. In particular, the EPA diet increased the ratio of EPA in the phospholipid fraction markedly (>20-fold) compared with the control group, which exhibited EPA levels below the detection limit. The EPA content in the brains of the EPA-enriched group was approximately twice as high as that of the DHA-enriched group; however, DHA content was almost same between EPA- and DHA-enriched groups, probably because of the higher basal level of DHA content in the brain. When the same amount of EPA and DHA is ingested, EPA appeared to be more effective than DHA in increasing its content in brain membranes. These results are essentially consistent with those reported in a previous study [Citation24].
Results of detailed analysis of changes in fatty acid composition in the brain due to intake of an EPA-enriched diet demonstrated that, not only EPA, but also relative amounts of other n-3 PUFA were increased. The proportion of long-chain n-3 fatty acids (22: 5, 22: 6) contained in EPA-40 was increased, which resulted in a decrease in the relative content of n-6 PUFA. In addition, the fold increase in EPA content was larger in the phospholipid fraction than in the total lipid fraction. It was confirmed again from this result that ingested EPA is taken up effectively in brain phospholipids. The n-3 PUFA of the cell membrane produce an eicosanoid with anti-inflammatory action [Citation27,28]. The observation that the effect of n-3 PUFA was noticeable under stress conditions indicated the possibility that n-3 PUFA are particularly important during inflammation [Citation4]. Although we did not investigate the effect of social isolation on brain fatty acid composition in the present study, it has been reported that social isolation does not affect fatty acid composition in the brain [Citation15].
The mechanism by which fish oil influences emotional behavior remains unclear. Accordingly, the exact mechanism by which EPA intake inhibited increases in anxiety-like behavior due to social isolation stress in this study was not elucidated. Regarding the mechanism of the effect of n-3 PUFA on emotional behavior, promotion of neuronal proliferation [Citation29], increase in monoamine concentrations in the brain [Citation30–32], and influence on brain-derived neurotrophic factor levels [Citation33,34] have been proposed. Because EPA had a particularly stronger effect than DHA in the present study, we believe that the anti-inflammatory effects of EPA play an important role. Prostaglandins derived from EPA have been reported to have anti-inflammatory properties [Citation35]. Moreover, resolvin E1 derived from EPA has been reported to decrease tumor necrosis factor (TNF)-α levels [Citation36]. Because resolvin E1 is associated with psychiatric diseases and inflammatory cytokines such as interleukin (IL)-1, IL-6, and TNF-α [Citation37], EPA ingestion possibly increases prostaglandin and/or resolvin E1 levels and reduces inflammatory reactions due to stress, which results in reduced anxiety-like behavior.
In conclusion, this study revealed that EPA has a stronger inhibitory effect on anxiety-like behaviors than DHA in rats under stress. It demonstrates the possibility of using EPA for emotional control in stressful situations.
Author Contribution
All members conceived and designed the experiments. Yasuyo Oshima performed the experiments. Yasuyo Oshima, Tasuku Watanabe, and Shun Endo analyzed data. Asako Takenaka wrote the paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This study was supported, in part, by the Ministry of Education, Culture, Sports, Science and Technology of Japan under Grant-in-Aid for Scientific Research [grant number 24580202].
References
- Hibbeln JR. Fish consumption and major depression. The Lancet. 1998;351(9110):1213.10.1016/S0140-6736(05)79168-6
- Li F, Liu X, Zhang D. Fish consumption and risk of depression: a meta-analysis. J Epidemiol Community Health. 2016;70(3):299–304.10.1136/jech-2015-206278
- Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: Evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006;67(12):1954–1967.10.4088/JCP.v67n1217
- Appleton KM, Grippo AJ, Beltz TG, et al. Consumption of a high n-3 polyunsaturated fatty acid diet during gradual mild physiological stress in rats. Prostaglandins Leukot. Essent. Fatty Acids. 2015;95:11–18.10.1016/j.plefa.2014.11.010
- Hallahan B, Ryan T, Hibbeln JR, et al. Efficacy of omega-3 highly unsaturated fatty acids in the treatment of depression. Brit J Psychiat. 2016;209(3):192–201.10.1192/bjp.bp.114.160242
- Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis. 2017;6(21):1–19.
- Gorman JM. Comorbid depression and anxiety spectrum disorders. Depression and Anxiety. 1996;4(4):160–168.10.1002/(ISSN)1520-6394
- Buydens-Branchey L, Branchey M, Hibbeln JR. Associations between increases in plasma n-3 polyunsaturated fatty acids following supplementation and decreases in anger and anxiety in substance abusers. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(2):568–575.10.1016/j.pnpbp.2007.10.020
- Liu JJ, Galfalvy HC, Cooper TB, et al. Omega-3 polyunsaturated fatty acid (PUFA) status in major depressive disorder with comorbid anxiety disorders. J Clin Psychiatry. 2013;74(7):732–738.10.4088/JCP.12m07970
- Mathieu G, Oualian C, Denis I, et al. Dietary n-3 polyunsaturated fatty acid deprivation together with early maternal separation increases anxiety and vulnerability to stress in adult rats. Prostaglandins Leukot. Essent. Fatty Acids. 2011;85(3–4):129–136.10.1016/j.plefa.2011.07.001
- Carlezon WA, Mague SD, Parow AM, et al. Antidepressant-like effects of uridine and omega-3 fatty acids are potentiated by combined treatment in rats. Biol Psychiatry. 2005;57(4):343–350.10.1016/j.biopsych.2004.11.038
- Ferraz AC, Delattre AM, Almendra RG, et al. Chronic ω-3 fatty acids supplementation promotes beneficial effects on anxiety, cognitive and depressive-like behaviors in rats subjected to a restraint stress protocol. Behav Brain Res. 2011;219(1):116–122.10.1016/j.bbr.2010.12.028
- Belzung C, Leguisquet AM, Barreau S, et al. Alpha-linolenic acid deficiency modifies distractibility but not anxiety and locomotion in rats during aging. J Nutr. 1998;128:1537–1542.
- Fedorova I, Salem N. Omega-3 fatty acids and rodent behavior. Prostaglandins Leukot. Essent. Fatty Acids. 2006;75(4–5):271–289.10.1016/j.plefa.2006.07.006
- Harauma A, Moriguchi T. Dietary n-3 fatty acid deficiency in mice enhances anxiety induced by chronic mild stress. Lipids. 2011;46(5):409–416.10.1007/s11745-010-3523-z
- McNamara RK. Role of omega-3 fatty acids in the etiology, treatment, and prevention of depression: Current status and future directions. J Nutr Intermed Metab. 2016;5:96–106.10.1016/j.jnim.2016.04.004
- Nakashima Y, Yuasa S, Hukamizu Y, et al. Effect of a high linoleate and a high alpha-linolenate diet on general behavior and drug sensitivity in mice. J Lipid Res. 1993;34:239–247.
- Francès H, Monier C, Bourre JM. Effects of dietary alpha-linolenic acid deficiency on neuromuscular and cognitive functions in mice. Life Sci. 1994;57:1935–1947.
- Chalon S, Delion-Vancassel S, Belzung C, et al. Dietary fish oil affects monoaminergic neurotransmission and behavior in rats. J Nutr. 1998;128:2512–2519.
- Carrié I, Clément M, de Javel D, et al. Phospholipid supplementation reverses behavioral and biochemical alterations induced by n-3 polyunsaturated fatty acid deficiency in mice. J Lipid Res. 2000;41:473–480.
- Mizunoya W, Ohnuki K, Baba K, et al. Effect of dietary fat type on anxiety-like and depression-like behavior in mice. SpringerPlus. 2013;2:165.10.1186/2193-1801-2-165
- Song C, Leonard BE, Horrobin DF. Dietary ethyl-eicosapentaenoic acid but not soybean oil reverses central interleukin-1-induced changes in behavior, corticosterone and immune response in rats. Stress. 2004;7(1):43–54.10.1080/10253890410001667188
- Song C, Zhang XY, Manku M. Increased phospholipase A2 activity and inflammatory response but decreased nerve growth factor expression in the olfactory bulbectomized rat model of depression: effects of chronic ethyl-eicosapentaenoate treatment. J Neurosci. 2009;29(1):14–22.10.1523/JNEUROSCI.3569-08.2009
- Ross BM, Malik I, Babay S. Dietary omega-3 polyunsaturated fatty acid supplementation in an animal model of anxiety. Prostaglandins Leukot. Essent. Fatty Acids. 2016;114:17–20.10.1016/j.plefa.2016.09.004
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(1):911–917.10.1139/y59-099
- Parker V, Morinan A. The socially-isolated rat as a model for anxiety. Neuropharmacology. 1986;25(6):663–664.10.1016/0028-3908(86)90224-8
- Wall R, Ross RP, Fitzgerald GF, et al. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutrition Rev. 2010;68(5):280–289.10.1111/nure.2010.68.issue-5
- Whelan J. The health implications of changing linoleic acid intakes. Prostaglandins Leukot. Essent. Fatty Acids. 2008;79(3–5):165–167.10.1016/j.plefa.2008.09.013
- Kawakita E, Hashimoto M, Shido O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience. 2006;139(3):991–997.10.1016/j.neuroscience.2006.01.021
- Zimmer L, Delpal S, Guilloteau D, et al. Chronic n-3 polyunsaturated fatty acid deficiency alters dopamine vesicle density in the rat frontal cortex. Neurosci Lett. 2000;284(1–2):25–28.10.1016/S0304-3940(00)00950-2
- Kodas E, Galineau L, Bodard S, et al. Serotoninergic neurotransmission is affected by n-3 polyunsaturated fatty acids in the rat. J Neurochem. 2004;89(3):695–702.10.1111/jnc.2004.89.issue-3
- Vancassel S, Leman S, Hanonick L, et al. n-3 Polyunsaturated fatty acid supplementation reverses stress-induced modifications on brain monoamine levels in mice. J Lipid Res. 2008;49(2):340–348.10.1194/jlr.M700328-JLR200
- Rao JS, Ertley RN, Lee HJ, et al. n-3 Polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry. 2007;12(1):36–46.10.1038/sj.mp.4001888
- Ferreira CF, Bernardi JR, Krolow R, et al. Vulnerability to dietary n-3 polyunsaturated fatty acid deficiency after exposure to early stress in rats. Pharmacol Biochem Behav. 2013;107:11–19.10.1016/j.pbb.2013.03.006
- Babcock T, Helton WS, Espat NJ. Eicosapentaenoic acid (EPA): an antiinflammatory ω-3 fat with potential clinical applications. Nutrition. 2000;16(11–12):1116–1118.10.1016/S0899-9007(00)00392-0
- Arita M, Yoshida M, Hong S, et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci USA. 2005;102(21):7671–7676.10.1073/pnas.0409271102
- Brietzke E, Kapczinski F. TNF-α as a molecular target in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1355–1361.10.1016/j.pnpbp.2008.01.006
