Abstract
Threshold of Toxicological Concern (TTC) decision-support methods present a pragmatic approach to using data from well-characterized chemicals and protective estimates of exposure in a stepwise fashion to inform decisions regarding low-level exposures to chemicals for which few data exist. It is based on structural and functional categorizations of chemicals derived from decades of animal testing with a wide variety of chemicals. Expertise is required to use the TTC methods, and there are situations in which its use is clearly inappropriate or not currently supported. To facilitate proper use of the TTC, this paper describes issues to be considered by risk managers when faced with the situation of an unexpected substance in food. Case studies are provided to illustrate the implementation of these considerations, demonstrating the steps taken in deciding whether it would be appropriate to apply the TTC approach in each case. By appropriately applying the methods, employing the appropriate scientific expertise, and combining use with the conservative assumptions embedded within the derivation of the thresholds, the TTC can realize its potential to protect public health and to contribute to efficient use of resources in food safety risk management.
INTRODUCTION
The Threshold of Toxicological Concern (TTC) describes a level of exposure to a defined grouping of classes of chemicals that is likely to be without harm, such that chronic exposures below the threshold can be assumed to be without appreciable risk over a lifetime. The concept of a threshold of exposure below which the risk to health is of limited concern is increasingly important given the ongoing advances in analytical chemistry that result in progressively lower detection limits. With lower limits, the number of different substances that can be detected increases (De Vries, Citation2006), leading to the discovery in food of small quantities of environmental contaminants, pesticide residues, natural toxins, packaging migrants, processing-induced chemicals (e.g., Maillard reaction products), and inadvertent contamination from processing equipment (Institute of Food Technologists, Citation2009).
Proper application of the TTC approach benefits regulators, producers, and consumers, as it allows resources to be dedicated to substances posing a greater threat to human health. The TTC is a tool that supports resource-efficient safety assessment of chemical contaminants. The upper bound risk nature of its derivation and proper application by experts can promote the protection of public health. Note that the term upper bound risk is used here to refer to calculations, scientific assumptions, and estimates that tend to overestimate the risks to public health through assumptions of greater toxicity and higher estimates of consumer exposure.
In a recent opinion on the method, the European Food Safety Authority (EFSA) supported the use of the TTC approach for low-level exposures to impurities and their breakdown and reaction products in food additives, substances in food contact materials and their breakdown and reaction products, metabolites and degradation and reaction products of pesticide active substances, and trace contaminants in food (EFSA, Citation2012). This use is supported provided that the context is given careful consideration and that the exposure estimate is also conservative. The opinion also notes that it is not appropriate for situations requiring data to be submitted under European Union regulations (EFSA, Citation2012).
EFSA and the Joint Expert Committee on Food Additives (JECFA) of the World Health Organization and the Food and Agriculture Organization of the United Nations currently use the TTC concept to evaluate flavor additives. In this context, the TTC has facilitated the safety assessment of over 1200 flavoring agents (Renwick, Citation2004). In the United States, the legality of the TTC concept has been demonstrated by reference to the Federal Food, Drug, and Cosmetic Act as well as principles of statutory construction and case law (Hahn, Citation2010). A similar approach was applied to develop the Threshold of Regulation (TOR) used by the U.S. Food and Drug Administration (U.S. FDA) to evaluate potential exposures arising from the migration of noncarcinogenic chemicals into food from food contact materials (U.S. FDA, Citation2010a). The U.S. Environmental Protection Agency (Citation1999) has also employed a similar approach to reduce the number of pesticides for which tolerances (maximum residue levels) must be established, stating that a tolerance need not be devised for a pesticide with no detectable residues and for which the “estimated potential risks of any theoretically possible residues in food is not a concern.” In each of these uses of a TTC approach in regulatory risk management, knowledge of worst-case risk from many chemicals provides a protective scientific basis for determining where to focus resources in situations in which very low levels of exposure to similar chemicals may occur.
The implementation of the TTC concept by some regulatory bodies and the apparent simplicity of the tiers and thresholds of the approach make it an attractive tool and vulnerable to potential misuse. The discussion presented herein is designed to help the risk manager determine whether the TTC approach is appropriate in a given situation. Because the TTC concept itself has been referred to as a “screening” method to prioritize toxicity testing and risk management measures (Felter et al., Citation2009), this paper outlines a prescreening process to be undertaken in response to the detection of an unexpected chemical in a food product, which will indicate whether the TTC approach may be appropriate.
To provide an understanding of the theory underlying the concept and the reason for current exclusions for its use for certain chemical groups, this paper begins with a summary of the scientific basis of the TTC. These excluded groups are described in more detail, along with other criteria that may render the TTC approach inappropriate. The issues involved in determining the applicability of the TTC approach for a contamination event are then described in stepwise fashion, and the information required for the approach is briefly discussed. Six case studies are introduced in which the steps are demonstrated in hypothetical situations. The scope of the discussion is limited to the decision on the applicability of the approach. This paper will not provide a specific TTC approach for any particular occurrence of a class of chemical or unknown analytical result in foods. For such approaches, the reader is referred to other more detailed analyses such as those by Felter et al. (Citation2009), Koster et al. (Citation2011), and Kroes et al. (Citation2004).
SCIENTIFIC BASIS OF THE TTC
Continuous improvements in the analytic sensitivity of detection have begun to reveal the complex, low-concentration chemical composition of many common foods and processed products. For example, marigold (Calendula officinalis) extract has been found to contain >150 named chemicals (Re et al., Citation2009), many of which are likely to be toxic if ingested in sufficient quantity. This substantial increase in the number of targets for safety assessment makes full dataset evaluations an untenable proposition from an animal testing perspective as well as from simple consideration of resources and timeliness.
In response to challenges and confusion related to regulatory oversight created by lists of permitted substances, Frawley (Citation1967) proposed the existence of a general threshold of exposure to food packaging materials below which no or only negligible harm might be expected. The general principle of the proposed approach is sometimes referred to as the de minimis concept. The term refers to a level of risk that is so small that it does not warrant further evaluation (Peterson, Citation2002). Frawley (Citation1967) estimated that de minimis risk for nonpesticide and nonheavy metal compounds would occur at 0.1 ppm (equivalent to approximately 150 μg/d), based on the distribution of no-effect levels for 220 two-year toxicity studies and the application of a 100-fold safety factor. Similarly, Rulis (Citation1986) initially estimated a de minimis risk of 0.15 μg/d based on a toxicity dataset of oral carcinogens. After further refinements (Munro, Citation1990; Gold et al., Citation1995), the U.S. FDA implemented the TOR at 0.5 ppb in food (U.S. FDA, Citation1995). The TOR corresponds to an intake of 1.5 μg/d. In addition to consideration of carcinogenicity chemical structural alerts, genotoxicity, and other required information, the TOR is deemed by the agency to be the level below which a contaminant in food would not be expected to have an appreciable adverse effect. The TOR is applied to food contact substances, in that a well-described substance in packaging or other food contact materials that is demonstrated to not migrate to food at levels >0.5 ppb is not subject to further toxicity data requirements as a food additive by the U.S. FDA (Shanklin, Citation2009). The substance is still considered to meet the regulatory and legal definition of the “reasonable certainty of no harm” statutory standard.
The fields of structure-activity relationships (SAR) and quantitative SAR (QSAR) overlap conceptually with TTC, and in fact are used within implementation of TTC approaches to allow refinement of the coverage of a particular TTC value to a defined class of chemicals. These SAR and QSAR exploit the tendency for chemicals in the same structural category to exhibit similarities with respect to physicochemical properties and human health, ecotoxicology, or environmental fate (Organization for Economic Cooperation and Development, Citation2011). Cramer et al. (Citation1978) made considerable progress on this front by devising a classification scheme to group compounds into classes associated with potential toxicity based on chemical structure. Munro et al. (Citation1996) applied the Cramer classification to a database of 611 organic chemicals from a wide range of applications, for which oral subchronic or chronic toxicity data existed for noncancer endpoints. This dataset has been subsequently quality reviewed (EFSA, Citation2012) and further evaluated through comparison with more recent datasets (CitationFraunhofer, N.D.). Munro et al. (1996) first estimated an intake level within each Cramer class by estimating the fifth percentile of the distribution of no-observed-effect levels (NOEL) in each class. They then divided the fifth percentiles by 100 to account for species differences and susceptible human populations, as is done for typical acceptable daily intake assessment for food additives, to derive a TTC value for each of the three Cramer classes. Munro et al. (1996) proposed that if exposures were below this level, the substance could be evaluated using fewer toxicological testing data because the most toxic fifth percentile of other similar chemicals did not show health effects at exposures 100-fold higher. A selection of TTC values derived in this manner is shown in . The combination of the selection of the fifth percentile no-observed-effect level and the use of conventional safety assessment factors provides the basis for considering the exposure threshold to be conservative (health protective).
Table 1 A selection of TTC values proposed in the scientific literature
The resulting threshold values can be applicable using expert judgment to very low-level chronic oral exposures to defined chemicals (considering defined exclusions) with only a minimal data requirement and consequently minimal animal testing. According to Munro et al. (Citation1996), these TTC values explicitly do not apply to the following groups: proteins, heavy metals, steroids, polyhalogenated dibenzo-p-dioxins and polyhalogenated dibenzofurans, and very high potency carcinogens such as azoxy- and N-nitroso-compounds and aflatoxin-like compounds. In most cases, these groups are excluded because they were not adequately represented in the original database on which the TTC values are based. To note, this list is not exhaustive and as databases are continually refined and new categories of chemicals or exposures are defined, additional thresholds may be derived (Van Ravenzwaay et al., Citation2011).
CONDITIONS NOT APPROPRIATE FOR THE TTC APPROACH
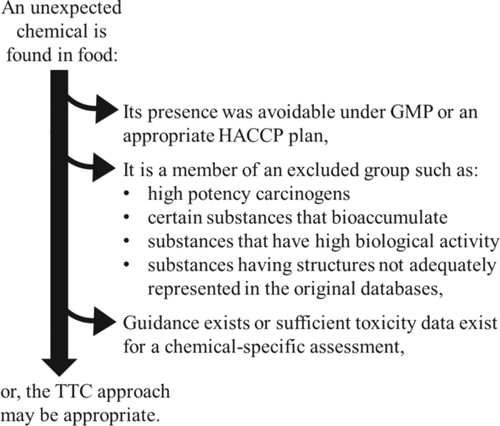
There are several groups of chemicals, defined by their structure or other specific data that are currently excluded from the TTC approach. However, this is not to say that the approach is appropriate in all other cases. The following situations represent conditions that disqualify a situation from being properly evaluated using the TTC concept, as summarized in .
Figure 1 Summary of the three main conditions indicating that the TTC approach is inappropriate to the situation.
The Contamination Event Was Avoidable Under Good Manufacturing Practices or an Appropriate Hazard Analysis and Critical Control Point (HACCP) Plan
Good manufacturing practices (GMP) describe procedures and conditions established in a food processing or storage facility that generally promote hygiene within the facility and processing line, such as regular hand washing, adequate ventilation, and maintenance of appropriate temperatures for food storage. They are a component of “prerequisite programs,” which must be in place before an effective Hazard Analysis and Critical Control Point (HACCP) plan is designed. An HACCP plan is a more tailored risk management plan that is designed and implemented by the manufacturer to control specific hazards that can be reasonably foreseen to pose a threat in that particular operation. GMP and HACCP plans are critical programs for the production of safe foods.
In the event of contamination of a food product, it is important to determine whether the contamination should have been prevented by effective GMP or HACCP procedures (i.e., whether it was reasonably foreseeable). Shortcomings in implementation or enforcement of food safety measures must be addressed within the best practice, guidance, or regulations for those measures. Such shortcomings render the use of the TTC approach invalid because it is not intended or designed to address contamination that results from the absence of appropriate control measures or from failure to enforce existing standards. For example, if the occurrence of a contaminant was foreseen in the HACCP plan for a food production process, then failures resulting in that contaminant's appearance in a food product should be appropriately addressed as specified by that HACCP plan and appropriately addressed (i.e., a failure cannot be allowed to continue because of a control measure not in the HACCP plan). Similarly, if a contamination is a result of a failure of GMP, then the failure must be corrected (i.e., the GMP failure cannot continue because the TTC was not exceeded). The TTC is a risk prioritization tool that must be reserved for those situations in which trace levels of a chemical occur in spite of adherence to GMP and applicable HACCP plans.
This point is consistent with the U.S. FDA (Citation2012) position on unavoidable contaminants in food as defined in Part 109 of Section 21 of the Code of Federal Regulations, which states that a tolerance (sufficient for the protection of public health) for a poisonous or deleterious substance may be established if the substance cannot be avoided by GMP, and if no improvements are anticipated in the near future that will affect this. However, such tolerance is not relevant for cases in which the contamination is avoidable.
The Chemical is a Member of an Excluded Group
Based on the current databases and evaluations supporting TTC, several classes of chemicals have been deemed inappropriate for the use of the TTC. These classes and the reasons for their exclusion are described below and summarized in .
Table 2 Classes of chemicals to which the TTC approach does not apply, and reasons for exclusion
High-Potency Carcinogens
A dataset of carcinogens based on the Gold Carcinogenic Potency Database was analyzed to find the proportion of chemicals in different structural groups that would yield an estimated lifetime risk >10−6 (one in a million) at a range of intake levels (Kroes et al., Citation2004). From this analysis, aflatoxin-like chemicals, azoxy compounds, and N-nitroso compounds were found to be the most potent of the groups studied, with a high proportion of members estimated to present an unacceptably high risk even with a daily intake of 0.15 μg. For this reason, these three groups of chemicals are excluded from the TTCs proposed in that paper, including the lowest TTC that was designed to apply to genotoxic carcinogens. Steroid hormones are also judged to be potent carcinogens as a class, although nongenotoxic, and are similarly excluded (Koster et al., Citation2011).
Certain Substances That Bioaccumulate
Kroes et al. (Citation2004) excluded certain substances on the basis of their ability to accumulate in the body because this makes the determination of a safe chronic daily intake particularly problematic. These substances include the polyhalogenated dibenzo-p-dioxins, polyhalogenated dibenzofurans, and polyhalogenated biphenyls, as well as some metals such as cadmium and lead.
Substances Not Adequately Represented in Training Databases
Several classes of substances were not represented in the original databases (Cramer et al., Citation1978; Gold et al., Citation1995; Munro et al., Citation1996; Cheeseman et al., Citation1999), including inorganic chemicals, metals, proteins, polymers, and unique structures. Proteins are also excluded by virtue of their potential allergenicity, an endpoint that is not considered in the derivation of current thresholds (EFSA, Citation2012). Thresholds derived from the original datasets cannot be assumed to apply to chemicals in classes that were not part of these datasets.
The Chemical is Well Characterized With Respect to Toxicity
The existence of toxicity data in sufficient quantity to permit a chemical-specific safety assessment suggests the need to use such data rather than apply a generic threshold such as the TTC. In some cases, this will already have been done, as evidenced by a maximum level, maximum residue level, acceptable or tolerable daily intake, or similar values derived by various scientific institutions and regulatory authorities at regional, national, or international levels. The existence of chemical-specific authoritative risk assessments overrides the application of the TTC. Note that development of such chemical-specific assessments is expected to generally result in higher (less restrictive) risk management decision values due to the multiple health protective assumptions in the derivation and use of the TTC decision approach when properly applied by experts. Specifically, the TTC decision approach uses no observed adverse effect levels (NOAELs) of the most toxic examples of well-studied chemicals structurally similar to the substance, safety adjustment factors (100-fold in the case of the 1996 TTC values by Munro et al.) to arrive at a “generic ADI” for the class of chemicals, expert evaluation of occurrence of well-known chemical-structure triggers for toxicity, and protective exposure estimates (e.g., Munro et al., Citation1996; Kroes et al., Citation2004; Felter et al., Citation2009; EFSA, Citation2012).
Table 3 Categories of chemicals found unexpectedly in food
STEPWISE DETERMINATION OF APPROPRIATENESS OF THE TTC APPROACH
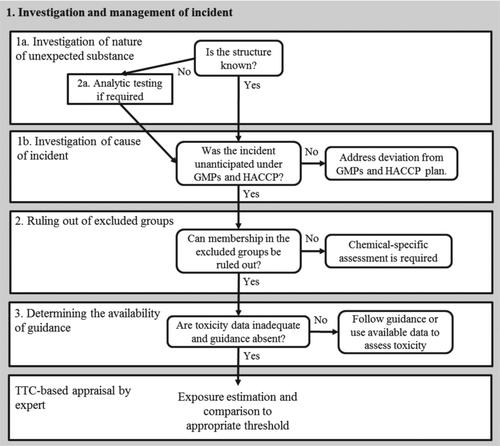
The following is intended to be applied by a person responsible for food safety evaluations in situations in which it is possible to encounter unexpected detections of chemicals that do not have full toxicity datasets. Such situations may arise in public health agencies monitoring the food supply or at a commercial food processing facility. Assuming that product testing has revealed the presence of an unexpected substance in the food, a number of determinations () made in the course of an investigation will inform the decision as to whether it is appropriate to have a toxicologist evaluate the contamination using the TTC approach. The steps are presented in sequence for clarity only, and with the exception of the investigation of cause, the order of their performance is not important.
Investigate and Manage the Incident
Among the first steps is to investigate the nature of the unexpected substance. In most cases in which chemical composition and structure data are available for the substance through standard chemical analysis methods, the identification of the material can be supplemented with online tools (e.g., ChemIDplus, http://sis.nlm.nih.gov/chemical.html). This information may be needed to determine whether sufficient data exist to enter into a particular expert-based TTC evaluation approach for the substance.
Investigate the Cause of the Incident
An investigation into the cause of the contamination should be initiated on first detecting the unexpected substance, and may be done concurrently with the remaining steps. Determination of the cause may be easier if the chemical can first be identified because the identity can provide indication as to the time and location of the contamination ().
After the cause has been determined, the avoidability of the contamination event needs to be considered. The composition of some natural ingredients (including toxins) can vary with genotypic and phenotypic variation, geographic origin, weather, harvesting practices, and processing conditions (Betz et al., Citation2011), and may occur in food independently of manufacturing practices. In contrast, contamination incidents resulting from avoidable error that could reasonably have been predicted, are within the scope of risk management measures embodied in the current GMP of the U.S. FDA (Citation2010b) or analogous HACCP plans or programs, such as Australian food safety standards of Food Standards Australia New Zealand (Citation2011).
When a chemical occurrence in a food product results from an unanticipated event that is not covered by GMP or recognized within a HACCP plan, the evaluation of potential risk associated with the chemical may be suited for the TTC approach. The key criterion in this case is that the TTC is not being used in lieu of the implementation of GMPs or of a comprehensive HACCP plan; it can be considered only after confirming that all relevant food safety measures are fully implemented in the processing chain and the facility at large. Furthermore, once detected, the occurrence of the chemical must be addressed through corrective actions.
Rule Out Excluded Groups
In this step, the determination must be made as to whether the chemical in question is a member of the excluded groups as described above (). If the chemical is a member of an excluded group, then the TTC does not apply. To note, maximum tolerable levels have already been set for many chemicals in the excluded groups.
In some cases, the identification of the chemical structure of the unexpected substance is not possible. As such, the TTC approach may still be appropriate if properties such as boiling point, water solubility, the octanol-water coefficient log P, Henry's Law constant, atmospheric OH rate constant, pKa dissociation constant, and vapor pressure are available as long as the substance can be shown not to belong to one of the TTC excluded groups () (see Koster et al. (Citation2011) for a detailed description of the potential application of the TTC to unidentified substances).
Determine the Availability of Guidance or Toxicity Data
The TTC approach fills a particular niche in the field of chemical risk assessment in which the available toxicity data are too few to perform a formal safety or risk assessment. Where official guidance has been elaborated, this should be followed. Otherwise, a determination needs to be made regarding whether existing toxicity data are sufficient to support a risk or safety assessment.
If the unknown contaminant has been identified, any existing national or international regulatory guidelines must be sought. The international standard, which is compatible with the Sanitary and Phytosanitary (SPS) Agreement of the World Trade Organization, is promulgated by the Codex Alimentarius Commission (Codex). The General Standard for Contaminants and Toxins in Food and Feed (Codex Standard 1993–1995) lists maximum levels for contaminants posing a potential health risk, such as mycotoxins, heavy metals, pesticides, veterinary drug residues, and others, in specific food products (Codex, Citation2012a,Citationb).
For products intended for distribution in particular countries, regional, national, or subnational guidelines may apply that are not consistent with a Codex standard. Such guidelines take precedence over a TTC approach. If in doubt, regulatory agencies often are repositories for such information. For example, the Chemical Safety Division of the U.K. Food Standards Agency (Citation2008) will provide advice as to the existence of relevant guidance values in the event of an incident in that country.
Flavorings are distinct from food additives under European Union regulations. In the European Union, the requirement to submit toxicity data for technically active substances in pesticides, additives in foods and feeds, and nutrient sources means that the TTC will not be applicable in these situations; however, it may apply to contaminants or breakdown products in these products (EFSA, Citation2012).
Determine Data Sufficiency for Formal Safety Assessment
In the event that no official guidance yet exists for the chemical, data from toxicology studies should be sought and evaluated as to their ability to support a formal safety assessment. Data on structurally related compounds may also be considered if they are sufficiently similar to the contaminant being assessed. Various online databases act as repositories of toxicology data for many potential contaminants (e.g., ToxNet.nlm.nih.gov). The risk manager should consult these to gain a general idea of the quantity of information available. It will ultimately be the responsibility of scientific specialists—such as experts in chemical SAR, exposure assessment, toxicology, epidemiology, or risk assessment—to determine whether there are data of sufficient quality to develop a defensible chemical-specific assessment of safety.
ELEMENTS OF A TTC-BASED APPRAISAL BY AN EXPERT
If none of the disqualifying conditions described above applies, qualified experts should be employed to implement the TTC approach by following the particular decision tree of the TTC approach they have chosen and choosing the correct threshold value to be applied (e.g., see Kroes et al., Citation2004; Felter et al., Citation2009). Specialist expertise is also required in the derivation of an appropriately constructed and sufficiently conservative exposure estimate.
Exposure Estimation and Comparison of Exposure to the Appropriate Threshold
Human dietary exposure to food chemicals is a function of both the concentration of the chemical per unit of food and the consumption pattern of that food among the population. For the chronic exposures for which the TTC approach was developed, the primary consideration is the average daily intake of the food per individual consumer (rather than the magnitude of any one-time intake). When the affected food is a commodity or other form of ingredient rather than an individual product, the total intake across all downstream uses of the commodity is relevant.
To confer protection on the majority of the population of consumers, the intake at a high percentile of the distribution across consumers should be used to calculate exposure. The average daily consumption of the food in kilograms is multiplied by the concentration of the chemical in the food to obtain the daily intake. This can be compared with a TTC value expressed in micrograms per day or in micrograms per kilogram of body weight per day.
Possible Refinements
Given that the TTC values, excluded categories, and exposure estimates used in TTC decision frameworks incorporate protective or upper bound risk assumptions, exceeding a threshold value does not necessarily imply a risk to health. If this result is obtained, there may be justification in considering mitigating factors such as negative mutagenicity data or short-term exposure (Felter et al., Citation2009; European Medicines Agency, Citation2010). In addition, the exposure estimate can be refined. If the initial exposure estimate was based on very conservative approximations in the absence of information, more accurate data can be sought that may demonstrate that a more refined exposure estimate (yet still applying the same high percentile of the consuming population's intake distribution) falls below the relevant threshold.
These and other science-based decisions will be made by appropriate experts in toxicology and exposure assessment specific to the conditions being considered. The result will be a decision given the stated assumptions about sampling reliability, analytic sensitivity, and exposure, showing that either the presence of the chemical is unlikely to result in appreciable risk of harm under the anticipated conditions or that there is insufficient confidence that the foregoing is true. This analysis will then be relayed to the risk manager, who then decides whether to accept or reject the product or to consider acquiring more information to assist with the decision.
It is critically important that the TTC not be misapplied. It must not be used as a rationale for neglecting to address and correct potential sources of chemical risks in products. In every case, the transparency of assumptions, calculations, and data sources should be documented and retained as a matter of good scientific practice and for legal and regulatory purposes.
CASE STUDIES: EXAMPLES USING THE DECISION CRITERIA
The TTC is designed for application to low-level exposures to chemicals; thus, simple understanding of the concentration in food is the first level of consideration of whether the TTC approach will be useful. For example, the TTC threshold value in of 90 μg/d for Cramer class III corresponds to a chemical concentration of 60 ppb (or 60 μg/kg) in food assuming that 1.5 kg of food is consumed at that concentration in a day. By the same argument, the TTC for Cramer class I is associated with a level in food of 1.2 ppm. Levels below these values, when considered in conjunction with accurate exposure data, are likely to be in the range in which the TTC is a practical approach. The case studies below address a series of situations of such low-level exposures that demonstrate determinations as to the applicability of the TTC approach.
Pesticide Residue
A lot of coffee beans arriving at the processing facility was found to contain permethrin at a level of 60–80 ppb. Permethrin is a synthetic pesticide derived from a pyrethrin found in chrysanthemum flowers, and is used to control thrips, aphids, and other insect pests.
Records from the grower indicated that an acceptable spray schedule had been followed, including an appropriate days-to-harvest interval, and the unexpected detection of permethrin was not the result of a GMP violation nor of a violation of good agricultural practice or of noncompliance with a HACCP plan. Permethrin is not a member of the excluded groups; however, Codex (Citation2012b) has established a maximum residue level for this pesticide. Given the availability of official guidance, the application of the TTC approach is inappropriate.
Environmental Containment
A test of a sample of frozen mixed seafood revealed the presence of decabromodiphenyl ether (decaBDE) at a level of 0.5 ppb. Further testing indicated that the contamination was associated with catfish that comprised 20% of the mixture.
This compound is a congener of the class of polybrominated diphenyl ethers (PBDEs). PBDEs were developed for use as flame retardants and originally considered immobile in the products in which they were used. However, as analytical detection methods improved, it was recognized that they have migrated to the environment and bioaccumulated in food chains (Alcock and Busby, Citation2006). Increasing levels have been found in human tissues, causing concern due to an association with endocrine disruption, reproductive toxicity, and cancer (Schecter et al., Citation2004).
The chemical is an environmental contaminant and its presence was not due to a gross GMP violation. Testing of fish at the site of production (an aquaculture operation) had been performed as per the HACCP plan; however, it did not show contamination at that time, nor did the land use of the surrounding area indicate potential for contamination. However, the potential of decaBDE to bioaccumulate means that it is classed within the excluded groups and thus is not an appropriate candidate for the TTC approach.
Mycotoxin Formation
A lot of coffee beans was found to contain ochratoxin A at a level of 60–80 ppb, just prior to roasting. Ochratoxin A is a mycotoxin produced by common fungal species within the Penicillium and Aspergillus genera, and is found most often in cereals such as wheat and oats but may also be detected in coffee and wine. The main effect of ochratoxin A in humans is kidney toxicity (JECFA, Citation2007) and it has been classified as a probable human carcinogen (International Agency for Research on Cancer, Citation2002).
In investigating the cause of the detection, it was found that the beans obtained from the grower had been stored in a poorly ventilated room, causing increased mycotoxin formation and constituting a deviation from the applicable HACCP plan. On this basis alone, the application of the TTC cannot be considered. This case is also subject to disqualification from the TTC approach based on the existence of a provisional tolerable weekly intake for ochratoxin A (JECFA, Citation2007).
Volatile Fungal Metabolite
A musty smell associated with a lot of dry soup mix was traced to dried mushrooms from an external source that, on testing, were found to be contaminated with tribromoanisole at a level of 20 ppt, resulting in a level of 2 ppt in the soup mix.
Tribromoanisole is a fungal metabolite of 2,4,6-tribromophenol that is used as a pesticide and flame retardant in wooden pallets. This highly volatile chemical can contaminate products stored on such pallets, and can cause a musty taint detectable by humans at a level of 2 ppt in food and 0.02 ppt in water (Whitfield et al., Citation1987).
The contamination could not have been anticipated and did not result from a failure of GMP or a deviation from an HACCP plan. The chemical is not a member of an excluded group, and no official guidance was found for the substance, although a review of toxicity data was recently published (Koschier et al., Citation2011), which should be evaluated by a toxicologist. Thus, the contamination falls within the scope of the application of TTC decision support.
Contamination by a Lubricant
A leak resulted in the detection in a confectionery product (“gummi” candies) of a lubricant approved for incidental food contact. Of all of the components of the lubricant, one substance, alkylated diphenylamine, was judged to be of possible concern. As a result of the leak, 500 kg of candy contained alkylated diphenylamines at a concentration of 40 ppb.
Lubricants are composed mainly of base oil such as mineral oil augmented with thickening agents and smaller quantities of additives such as antioxidants and corrosion inhibitors. Alkylated diphenylamine functions as an antioxidant in lubricants approved for uses in which contact with food is not ruled out.
The equipment failure resulting in the release could not have been anticipated in the context of an HACCP plan and was not affected by GMP. The alkylated diphenylamines are not members of any excluded groups, and few toxicology data are available. This situation may be a good candidate for the application of the TTC approach.
Unidentified Chemical Formed During Processing
An unexpected peak was detected in the chromatograph of an extruded cracker-type product. The mean concentration is estimated at <50 ppb.
In reviewing the production history of the lot, no deviation from GMP or HACCP was found. The chemical could not be identified in initial tests; however, targeted testing according to the guidelines of Koster et al. (Citation2011) permitted the determination that the chemical was not a member of the excluded groups and contained no structural alerts consistent with being a potent carcinogen. Given that the substance is unknown, no toxicity data or official guidance apply. On this basis, the TTC approach is considered appropriate for this situation.
DISCUSSION
With continued advances in analytical chemistry techniques (Misiwa et al., Citation2010), more challenges can be expected in the area of discovering and evaluating the safety of very low levels of contaminants in food. Because the TTC approach offers the potential to greatly simplify the assessment and prioritization of chemical risks, it is important that the rigor, and thus the legitimacy, of the approach be maintained.
Although the set of TTC values has a misleading simplicity, each value is based on widely reviewed rigorous scientific principles applied to well-regarded toxicity data. Taken together, the resulting approach can help risk managers in the regulatory authorities and the food industry by permitting them to prioritize testing and allocation of resources to those situations in which the need (i.e., the potential for harm to health) is greatest.
Since the initial establishment of the tiered, structure-based TTC values developed by Munro et al. (Citation1996), some effort has gone into recognizing finer categories of toxicity, resulting in the publication of additional threshold values for more specific chemical classes () (Kroes et al., Citation2004; Felter et al., Citation2009). These refinements can present improved clarity in decision support by experts using the TTC. However, the granularity of the subdivisions of data and the number of TTC threshold values may have a natural value-added limit.
The challenge of evaluating the appearance of a chemical mixture in food, giving rise to a “forest of peaks” on a chromatograph, has not been addressed definitively. The European Medicines Agency (Citation2010) approach is to apply the relevant threshold independently to each component of the mixture for structurally unrelated genotoxic impurities in pharmaceuticals. In addition to chemical structure and mode of action, target organ and mechanism of action should be taken into account in assessing the possible additive (or synergistic) effects of contaminants (Boobis et al., Citation2011). Rennen et al. (Citation2011) recently developed a system of assessing chemically complex food matrices based on the decision scheme of Kroes et al. and used new thresholds derived from new analyses of the original databases. Ongoing research into toxicokinetic and toxicodynamic interactions may lead to refinements in uncertainty factors for chemical mixtures, which may in turn provide valuable insights into the proper application of the TTC concept to co-occurring contaminants (Dorne et al., Citation2009).
Currently, given the limitations on applicability, the TTC approach is anticipated to apply in only a small proportion of contamination events. Future advances in this field are expected in the area of the exposure duration and/or frequency, with the aim to extend the methodology to explicitly account for less than chronic exposures and to chemicals with the potential to bioaccumulate. In addition, consideration must be given to additional types of contaminants such as radionuclides and newer substances such as nanomaterials, with respect to whether a structure-based threshold can be devised in those cases.
When applied in accordance with the stepwise decision approach presented, the TTC concept fulfills a narrow but important role in chemical risk management. It provides science-based justification for focusing the need for animal use and investment in toxicity testing to those cases in which it provides the greatest benefit for public health. Animal use is not needed for exposures that are so low that the testing will not provide any benefit. The TTC approach is particularly well suited to address the continual advances in analytical chemistry sensitivity, because reductions in the limits of detection will create situations in which the relevant exposure is more likely to fall into the range covered by the TTC exposure threshold values.
ACKNOWLEDGMENTS
This work was supported by the Technical Committee on Food and Chemical Safety of the North American Branch of the International Life Sciences Institute (ILSI North America). Authors Greg Paoli and Margaret Wilson served as consultants to this project and received funds from the ILSI North America Technical Committee on Food and Chemical Safety for their work on this paper.
ILSI North America is a public, nonprofit foundation that provides a forum to advance understanding of scientific issues related to the nutritional quality and safety of the food supply by sponsoring research programs, educational seminars and workshops, and publications. ILSI North America receives support primarily from its industry membership.
REFERENCES
- Alcock , R. E. and Busby , J. 2006 . Risk migration and scientific advance: The case of flame-retardant compounds . Risk Anal. , 6 : 369 – 381 .
- Betz , J. M. , Brown , P. N. and Roman , M. C. 2011 . Accuracy, precision, and reliability of chemical measurements in natural products research . Fitoterapia , 82 : 44 – 52 .
- Boobis , A. , Budinsky , R. , Collie , S. , Crofton , K. , Embry , M. , Felter , S. , Hertzberg , R. , Kopp , D. , Mihlan , G. , Mumtaz , M. , Price , P. , Solomon , K. , Teuschler , L. , Yang , R. and Zaleski , R. 2011 . Critical analysis of literature on low-dose synergy for use in screening chemical mixtures for risk assessment . Crit. Rev. Toxicol. , 41 : 369 – 383 .
- Cheeseman , M. A. , Machuga , E. J. and Bailey , A. B. 1999 . A tiered approach to threshold of regulation . Food Chem. Toxicol. , 37 : 387 – 412 .
- Codex Alimentarius Commission . 2012a . Current official standards Available from http://www.codexalimentarius.net/web/standard_list.do?lang=en. Accessed May 16, 2012
- Codex Alimentarius Commission . 2012b . Pesticide residues in food and feed Available from http://www.codexalimentarius.net/pestres/data/pesticides/index.html. Accessed May 16, 2012
- Cramer , G. M. , Ford , R. A. and Hall , R. L. 1978 . Estimation of toxic hazard–A decision tree approach . Food Cosmet. Toxicol. , 16 : 255 – 276 .
- De Vries , J. W. 2006 . Chasing “zero” in chemical contaminant analysis . Food Safety Magazine , Available from http://foodsafetymagazine.com/article.asp?id=536&sub=sub1. Accessed May 16, 2012
- Dorne , J. L. C. M. , Bordajandi , L. R. , Amzal , B. and Ferrari , P. 2009 . Combining analytical techniques, exposure assessment and biological effects for risk assessment of chemicals in food . Trends Anal. Chem. , 28 : 695 – 707 .
- European Food Safety Authority . 2012 . Scientific Opinion on Exploring options for providing advice about possible human health risks based on the concept of Threshold of Toxicological Concern (TTC) Available from http://www.efsa.europa.eu/en/efsajournal/pub/2750.htm. Accessed November 2, 2012
- European Medicines Agency . 2010 . Questions and answers on the “Guideline on the limits of genotoxic impurities.” Available from http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002907.pdf. Accessed May 16, 2012
- Felter , S. , Lane , R. W. , Latulippe , M. E. , Llewellyn , G. C. , Olin , S. S. , Scimeca , J. A. and Trautman , T. D. 2009 . Refining the threshold of toxicological concern (TTC) for risk prioritization of trace chemicals in food . Food Chem. Toxicol. , 47 : 2236 – 2245 .
- Food Standards Australia New Zealand . 2011 . “ Food safety standards (Australia only) ” . In Food standards code Available from http://www.foodstandards.gov.au/foodstandards/foodstandardscode.cfm. Accessed May 16, 2012
- Fraunhofer . N.D. . RepDose: Database for the analysis of relationship between chemical function groups/categories and target organs in repeated dose studies . Available from http://www.fraunhofer-repdose.de/. Accessed May 18, 2012
- Frawley , J. P. 1967 . Scientific evidence and common sense as a basis for food packaging regulations . Food Chem. Toxicol. , 5 : 293 – 308 .
- Gold , L. S. , Manley , N. B. , Slone , T. H. , Garfinkel , G. B. , Ames , B. N. , Rohrbach , L. , Stern , B. R. and Chow , K. 1995 . Sixth plot of the carcinogenic potency database: Results of animal bioassays published in the general literature 1989–1990 and by the US NTP 1990–1993 . Environ. Health Perspect. , 103 : 3 – 122 .
- Hahn , M. J. 2010 . FDA has the legal authority to adopt a threshold of toxicological concern for substances in food at trace levels . Food Drug Law J. , 65 : 217 – 230 .
- Institute of Food Technologists . 2009 . Making decisions about the risks of chemicals in foods with limited scientific information . Comp. Rev. Food Sci. Food Safety , 8 : 269 – 303 .
- International Agency for Research on Cancer . 2002 . Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans , Vol. 82 , Lyon , , France : IARC .
- Joint Expert Committee on Food Additives . 2007 . Evaluation of Certain Food Additives and Contaminants: 68th Report of the Joint FAO/WHO Expert Committee on Food Additives , Geneva , , Switzerland : World Health Organization .
- Koschier , F. , Gallo , M. A. , Feng , X. , Baxter , G. E. , Preston , R. , Stevens , K. and Powers , W. 2011 . Toxicological studies on 2,4,6-tribromoanisole . Food Chem. Toxicol. , 49 : 2074 – 2080 .
- Koster , S. , Boobis , A. R. , Cubberly , R. , Hollnagel , H. M. , Richling , E. , Wildemann , T. , Würtzen , G. and Galli , C. L. 2011 . Application of the TTC concept to unknown substances found in analysis of foods . Food Chem. Toxicol. , 49 : 1643 – 1660 .
- Kroes , R. , Renwick , A. G. , Cheeseman , M. , Kleiner , J. , Mengelsdorf , I. , Piersma , A. , Schilter , B. , Schlatter , J. , van Schothorst , F. , Vos , J. G. and Wurtzen , G. 2004 . Structure-based thresholds of toxicological concern-guidance for application to substances present at low levels in the diet . Food Chem. Toxicol. , 42 : 65 – 83 .
- Misiwa , N. , Mitsuno , H. , Kansaki , R. and Takeuchi , S. 2010 . Highly sensitive and selective odorant sensor using living cells expressing insect olfactory receptors . Proc. Natl. Acad. Sci. U. S. A. , 107 : 15340 – 15344 .
- Munro , I. C. 1990 . Safety assessment procedures for indirect food additives: An overview . Regul. Toxicol. Pharmacol. , 12 : 2 – 12 .
- Munro , I. C. , Ford , R. A. , Kennepohl , E. and Sprenger , J. G. 1996 . Correlation of structural class with no-observed-effect levels: A proposal for establishing a threshold of concern . Food Chem. Toxicol. , 34 : 829 – 867 .
- Munro , I. C. , Renwick , A. G. and Danielewska-Nikiel , B. 2008 . The threshold of toxicological concern (TTC) in risk assessment . Toxicol. Lett. , 180 : 151 – 156 .
- Organization for Economic Cooperation and Development . 2011 . Assessment of chemicals Available from http://www.oecd.org/document/6/0,3746,en_2649_34379_43087494_1_1_1_1,00.html. Accessed May 16, 2012
- Peterson , M. 2002 . What is a de minimis risk? . Risk Manage. , 4 : 47 – 55 .
- Re , T. A. , Mooney , D. , Antignac , E. , Dufour , E. , Bark , I. , Srinivasan , V. and Nohynek , G. 2009 . Application of the threshold of toxicological concern approach for the safety evaluation of calendula flower (Calendula officinalis) petals and extracts used in cosmetic and personal care products . Food Chem. Toxicol. , 47 : 1246 – 1254 .
- Rennen , M. A. J. , Koster , S. , Krul , C. A. M. and Houlben , G. F. 2011 . Application of the threshold of toxicological concern (TTC) concept to the safety assessment of chemically complex food matrices . Food Chem. Toxicol. , 49 : 933 – 940 .
- Renwick , A. G. 2004 . Toxicology databases and the concept of thresholds of toxicological concern as used by the JECFA for the safety evaluation of flavoring agents . Toxicol. Lett. , 149 : 223 – 234 .
- Rulis , A. M. 1986 . “ De minimis and the threshold of regulation ” . In Food Protection Technology , Edited by: Felix , C. W. 29 – 37 . Chelsea , MI : Lewis Publishers Inc. .
- Schecter , A. , Päpke , O. , Tung , K. C. , Staskal , D. and Birnbaum , L. 2004 . Polybrominated diphenyl ethers contamination of United States food . Environ. Sci. Technol. , 38 : 5306 – 5311 .
- Shanklin , A. P. 2009 . Regulatory report. How FDA's “threshold of regulation” program works . Food Safety Magazine , Available from http://www.foodsafetymagazine.com/articleFF.asp?id=2719&sub=sub1. Accessed May 16, 2012
- U.K. Food Standards Agency . 2008 . “ Principles for preventing and responding to food incidents ” . In A guidance document produced by the Food Standards Agency's Taskforce on Incidents Available from http://www.food.gov.uk/multimedia/pdfs/incidentsprinciples.pdf. Accessed May 16, 2012
- U.S. Environmental Protection Agency . 1999 . Threshold of regulation policy—Deciding whether a pesticide with a food use pattern needs a tolerance Available from http://www.epa.gov/fedrgstr/EPA-PEST/1999/October/Day-27/6042.pdf. Accessed May 16, 2012.
- U.S. Food and Drug Administration . 1995 . Food additives; threshold of regulation for substances used in food-contact articles . Fed. Regist. , 60 : 36582 – 36596 . Available from: http://www.gpo.gov/fdsys/pkg/FR-1995-07-17/pdf/95-17435.pdf. Accessed May 21, 2012
- U.S. Food and Drug Administration . 2010a . Agency information collection activities; proposed collection; comment request; threshold of regulation for substances used in food-contact articles . Fed. Regist. , 75 : 18209 – 18210 . Available from http://www.federalregister.gov/articles/2010/04/09/2010-8050/agency-information-collection-activities-proposed-collection-comment-req-uest-threshold-of-regulation#h-8. Accessed May 16, 2012
- U.S. Food and Drug Administration . 2010b . Current good manufacturing practices (CGMPs) Available from http://www.fda.gov/Food/GuidanceComplianceRegulatoryInformation/CurrentGoodManufacturingPracticesCGMPs/default.htm. Accessed May 16, 2012
- U.S. Food and Drug Administration, Department of Health and Human Services . 2012 . “ Title 21 (Part 109)–Food and drugs. Subchapter B–Food for Human Consumption ” . In Part 109–unavoidable contaminants in food for human consumption and food-packaging material Available from http://ecfr.gpoaccess.gov/cgi/t/text/text-idx?c=ecfr&sid=70f8f746d2946610748907097e62eac5&tpl=/ecfrbrowse/Title21/21cfr109_main_02.tpl. Accessed May 16, 2012
- Van Ravenzwaay , B. , Dammann , M. , Buesen , R. and Schneider , S. 2011 . The threshold of toxicological concern for prenatal developmental toxicity . Reg. Toxicol. Pharmacol. , 59 : 81 – 90 .
- Whitfield , F. B. , Hill , J. L. and Shaw , K. J. 1987 . 2,4,6-tribromoanisole: A potential cause of mustiness in packaged food . J. Agric. Food Chem. , 45 : 889 – 893 .