Abstract
Carotenoids are isoprenoids widely distributed in foods that have been always part of the diet of humans. Unlike the other so-called food bioactives, some carotenoids can be converted into retinoids exhibiting vitamin A activity, which is essential for humans. Furthermore, they are much more versatile as they are relevant in foods not only as sources of vitamin A, but also as natural pigments, antioxidants, and health-promoting compounds. Lately, they are also attracting interest in the context of nutricosmetics, as they have been shown to provide cosmetic benefits when ingested in appropriate amounts. In this work, resulting from the collaborative work of participants of the COST Action European network to advance carotenoid research and applications in agro-food and health (EUROCAROTEN, www.eurocaroten.eu, https://www.cost.eu/actions/CA15136/#tabs|Name:overview) research on carotenoids in foods and feeds is thoroughly reviewed covering aspects such as analysis, carotenoid food sources, carotenoid databases, effect of processing and storage conditions, new trends in carotenoid extraction, daily intakes, use as human, and feed additives are addressed. Furthermore, classical and recent patents regarding the obtaining and formulation of carotenoids for several purposes are pinpointed and briefly discussed. Lastly, emerging research lines as well as research needs are highlighted.
Introduction
Carotenoids are widespread compounds in nature. They are biosynthesized by photosynthetic organisms (cyanobacteria, algae, plants) as well as by some fungi and bacteria. The vast majority of animals cannot biosynthesize carotenoids although carotenoids can be incorporated through the diet and modified structurally thereafter. However, it has been demonstrated that certain invertebrate animals, including hemipteran (aphids, adelgids, phylloxerids) and dipteran (gall midges) insects and mites, can synthesize carotenoids de novo (Rodríguez-Concepcion et al. Citation2018).
Since the 1980s, interest in carotenoids as possible health-promoting compounds has expanded considerably. Although the nutritional importance of provitamin A carotenoids is undeniable, the categorical demonstration of their importance to promote health is extremely challenging due to the complexity of the diet and of the human organism. However, there are different strands of evidence coming from diverse studies (epidemiological, chemical, lab animals, cell cultures, etc.) indicating that health benefits from their consumption as part of normal diets could be expected.
Thus, optimal carotenoid intakes may be related to reduced risks of developing certain cancers (cervical, ovarian, colorectal, prostate, breast), cardiovascular disease, bone, skin, or eye disorders. Moreover, recent works suggest that they may be important in relation to mental health, metabolic health, during pregnancy and early life and even provide cosmetic benefits (Meléndez-Martínez Citation2019; Meléndez-Martínez, Mapelli-Brahm, and Stinco Citation2018). Although the possible beneficial actions of carotenoids in humans are usually attributed to antioxidant mechanisms, it should be noted that there may be other mechanisms including pro-oxidant mechanisms, enhancement of gap junctional intercellular communication, modulation of signaling pathways, absorption of visible light or modulation of membrane properties, which may act in conjunction. On the other hand, evidence is accumulating that oxidative cleavage derivatives of carotenoids other than retinoids can be biologically active in humans and that they may be related to some of the health benefits attributed to carotenoids (Meléndez-Martínez Citation2019; Rodríguez-Concepcion et al. Citation2018).
In relation to the importance of dietary carotenoids in nutrition and health, and evolutionary aspects, it is noteworthy that humans and their immediate ancestors have always fed on green leaves and that these contain high amounts of β-carotene and lutein.
The former can be cleaved into vitamin A activity-exhibiting compounds, while the latter accumulates prominently in the macula lutea and the brain, among other tissues and fluids (Johnson Citation2014). Interestingly, lutein and other carotenoids also form part of the diet of newborns as they are secreted with the mothers’ milk. Indeed, the yellowish color of colostrum, the first food of breastfed babies maybe due to the higher concentration of carotenoids relative to the milk produced in later lactation (Johnson Citation2014; Sommerburg et al. Citation2000).
Main food carotenoids
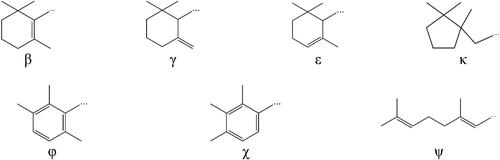
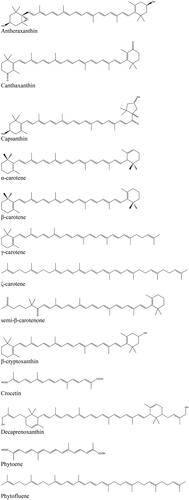
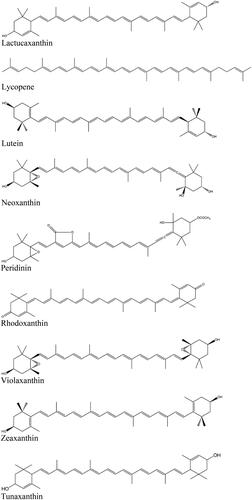
The majority of dietary carotenoids consumed by humans are obtained from plant derived foods. Carotenoids present in human diets typically contain C40 skeleton (tetraterpenoids), although there are some examples with a lower carbon number, such as apocarotenoids. Taking into account the usual food commodities present in the daily diet, humans have access to about 50 carotenoids. However, in human blood plasma, the number of carotenoids is reduced to six major ones, namely, α-carotene, β-carotene, lycopene, β-cryptoxanthin, zeaxanthin, and lutein () as well as the long ignored colorless carotenoids phytoene and phytofluene () (Meléndez-Martínez et al. Citation2015). Determination of the carotenoid contents in foods has been the main objective of many studies, and the resulting data have been compiled in databases and other food carotenoid compilations, as it will be discussed in another section. There is an increasing interest in searching new natural sources for carotenoids (e.g., underutilized wild fruits and vegetables), as well as in the selection, breeding, and enhancement of traditional cultivars of well-known staple food (potato, maize, wheat, etc.) (Atienza et al. Citation2007; Brown Citation2008; Murillo, Meléndez-Martínez, and Portugal Citation2010; De Rosso and Mercadante Citation2007; Shewry and Hey Citation2015).
Figure 2. Chemical structures of diverse carotenoids.
Fruit and vegetables are considered the most important sources for carotenoids in the human diet (Britton and Khachik Citation2009). However, the contribution of animal-derived food must not be overlooked, as egg yolk, dairy products (milk, butter, etc.) and seafood may provide a significant amount of certain carotenoids (e.g., lutein, zeaxanthin, astaxanthin, and canthaxanthin).
The distribution of carotenoids among the different higher plants does not obey a single pattern (Britton and Khachik Citation2009; Mínguez-Mosquera, Hornero-Méndez, and Pérez-Gálvez Citation2008). In green plant tissues (leaves, stems, seeds, and unripe fruits) carotenoids are located in the chloroplasts where they are associated with chlorophylls. Remarkably, the carotenoid profile in chloroplasts is very much conserved, consisting of one major carotene (β-carotene, 25%–30%) and three xanthophylls (lutein, 40%–50%, violaxanthin, 15% and neoxanthin, 15%). Other minor carotenoids (α-carotene, γ-carotene, β-cryptoxanthin, zeaxanthin, antheraxanthin, and lutein 5,6-epoxide) are also found in green vegetables. In contrast, in fruits, tubers and some seeds, carotenoid pigments, and especially the xanthophylls, are normally found in greater amounts, presenting a wider range of functional groups in their structure. The chromoplasts are the organelles specialized in the massive accumulation of carotenoids present in ripe fruits, and certain roots and tubers. The transformation from chloroplast to chromoplast is associated with the fruit ripening process and is characterized by a massive synthesis of carotenoids, which is usually accompanied by a change in the carotenoid profile of the fruit. Whereas green leaves contain free hydroxy-xanthophylls (unesterified), the native form of most xanthophylls in ripe fruits is as fatty acids esters (frequently mono- and diesters), hence the expanding interest in the analysis and study of carotenoid esters in foods (Hornero-Méndez Citation2019; Mariutti and Mercadante Citation2018).
Britton and Khachik (Citation2009) have proposed five distinctive carotenoid patterns in relation to the color of the plant tissue:
large amounts of the acyclic carotene lycopene, as in tomatoes (red color);
large amounts of β-carotene and/or its hydroxyl derivatives β-cryptoxanthin and zeaxanthin (orange color);
similar to pattern 2 but presenting also α-carotene and/or its hydroxyl derivatives, especially lutein (yellow-orange color);
large amounts of carotenoid epoxides (yellow color); and
carotenoids that appear to be unique to or characteristic of that species (yellow, orange, or red color), e.g., capsanthin and capsorubin in red peppers, and crocetin in saffron.
In relation to the first pattern, it is to be noted that lycopene is usually accompanied by the colorless carotenoids phytoene and phytofluene (Dias et al. Citation2018; Meléndez-Martínez et al. Citation2015).
Carotenoid analysis
The general procedure for the determination of carotenoids in different matrices can be divided into the following steps: sample preparation, extraction and saponification followed by separation, identification and quantification of the carotenoids. Among other factors, carotenoids are very sensitive to heat, light, oxygen, and acids resulting in some degree of degradation and/or isomerization. Consequently, precaution must be taken throughout the analysis to minimize the possible loss of carotenoids and thereby achieve reliable data. Analysis of certified reference material is the preferred procedure for verifying method performance; the analytical process from extraction to instrumental measurement can be assessed and for carotenoids in freeze-dried mixed vegetables a certified reference material has been developed: Community Bureau of Reference BCR485. Moreover, the European Committee for Standardization (CEN) has validated some methods of analysis for the determination of astaxanthin, canthaxanthin and β-carotene in food: CEN/TC 275/WG9. An example there is EN 12823-2:2000 (Foodstuffs—Determination of vitamin A by high performance liquid chromatography—Part 2: Measurements of beta-carotene).
Food sampling
It is essential to collect samples that are representative of the market in the specific countries. The collected foods should be main contributors to the total intake of carotenoids either by being consumed in high amounts and/or by containing very high levels of carotenoids. Foods are biological materials. Consequently, there is a natural variation in the composition of carotenoids in foods. Many factors influence the content of carotenoids and considerations about cultivation, seasonal variation, handling during harvest, and storage as well as processing and cooking parameters should be included in the sampling plan. If the purpose of a study is to estimate the extent of the natural variation, samples must be analyzed separately. If not, samples can be pooled and analyzed to get an average assessment of the content of carotenoids in the composite samples. More information about sampling for carotenoid analysis can be found elsewhere (De Rosso and Mercadante Citation2007; Rodríguez-Amaya and Kimura, Citation2004).
Sample preparation
To minimize the possible loss of carotenoids, quick handling of samples with a minimum exposure to heat, light, and oxygen must be performed. The first step of the sample preparation is to separate the edible and inedible material from each other, e.g., to peel oranges and to remove the inner stem from cabbage. Most foods are heterogeneous and it is optimal to freeze-dry the edible part of the samples before homogenization to ensure optimal homogenization and weighing of a representative part of the samples for analyses. Due to practical issues, it might be necessary to take out representative parts of the foods, for instance quarters of cabbage heads, and snap freeze them in liquid nitrogen to stop metabolic reactions before freeze-drying. The samples should be analyzed as quickly as possible after homogenization. If storage is necessary, the samples should be stored in a freezer preferably at −40 °C or lower under vacuum or an inert atmosphere. It is difficult to predict the storage time because of the individual influence and interplay of many factors. More information about sample preparation for carotenoid analysis can be found elsewhere (De Rosso and Mercadante Citation2007; Rodríguez-Amaya and Kimura Citation2004).
Extraction and saponification
Various techniques have been used for extraction of carotenoids. Liquid-liquid extraction is the traditional extraction method. However, numerous more recently developed extraction techniques have been described and reviewed elsewhere. These include ultrasound assisted extraction (UAE), microwave assisted extraction (MAE), enzymatically assisted extraction (EAE), pressurized liquid extraction (PLE), also known as accelerated solvent extraction (ASE), and supercritical fluid extraction (SFE) (Mustafa and Turner Citation2011; Saini and Keum Citation2018; Singh, Ahmad, and Ahmad Citation2015; Strati and Oreopoulou Citation2014; Xu et al. Citation2017).
Selection of solvents is one of the most important factors in carotenoid analyses. The optimal combination of solvents depends on the complexity of the food matrices and the polarity of the selected carotenoids (carotenes/xanthophylls). Different solvents or mixtures of these have been used for extraction of carotenoids over the years, e.g., acetone, tetrahydrofuran, petroleum ether, diethyl ether, chloroform, hexane, ethyl acetate, and ethanol. The presence of antioxidants is recommended to protect the carotenoids from oxidation during extraction. The most often added antioxidant is butylated hydroxytoluene (BHT) (Amorim-Carrilho et al. Citation2014). Sometime, samples are analyzed after the addition of a known amount of an internal standard (IS) which exhibits similar chemical properties but is easily distinguished from the analyte, and then the concentration of carotenoids in the sample extract is determined by relating the area ratio of each carotenoid and that of the IS to those of the calibration curves.
After extraction, the next step in the analysis of carotenoids is most often alkaline saponification, where any xanthophyll esters, e.g. present in many fruits, are hydrolyzed. Additionally, unwanted components like triacylglycerols and chlorophylls are removed. The purpose of removing triacylglycerols and chlorophylls is to avoid interference in separation, detection and quantification. There is no need to saponify food samples with low levels of all these compounds (Rodríguez-Amaya Citation2010). Recently, analytical methods without saponification have been developed to identify and quantify the native carotenoid composition of foods including both free and ester forms. These methods have been reviewed by Mercadante et al. (Citation2017).
Carotenoid separation, identification, and quantification
High-performance liquid chromatography (HPLC) has been, and still is, extensively applied to carotenoid separation. Improved efficiency in carotenoid characterization has been reported on C18 column using rapid resolution liquid chromatography (RRLC) (Stinco et al. Citation2014, Citation2018) and ultra high-performance liquid chromatography systems (UHPLC) (Amorim-Carrilho et al. Citation2014; Bijttebier et al. Citation2014; Herrero et al. Citation2008; Rivera and Canela-Garayoa Citation2012). The application of reversed-phase C30 columns to the separation of carotenoid isomers was firstly reported in 1994 (Sander et al. Citation1994), and due to the enhanced separation power of this type of stationary phase for the carotenoids, resulting from higher hydrophobic interactions taking place compared to the C18 one, it has become a commonly utilized stationary phase in carotenoid analysis. The serial connection of different columns has been proposed as an alternative to one single column LC (Dugo, Herrero, Giuffrida, et al. Citation2008). Multidimensional liquid chromatography (2 D-LC) has also been proposed and applied to carotenoid analysis in those cases where the sample matrix was very complex, in both on-line (Cacciola et al. Citation2012, Citation2016; Dugo, Herrero, Kumm, et al. Citation2008) and off-line approaches (Bonaccorsi et al. Citation2016). Supercritical fluid chromatography (SFC) coupled to mass spectrometry has lately attained consideration as a rapid, green and convenient technology applied to carotenoid analysis (Jumaah et al. Citation2016; Li et al. Citation2015), and only very recently the direct online extraction and determination of carotenoids, by a supercritical fluid extraction-supercritical fluid chromatography-mass spectrometry (SFE-SFC-MS) methodology was reported (Zoccali et al. Citation2017), and a supercritical fluid chromatography-triple quadrupole/mass spectrometry methodology for apocarotenoids determination was also lately available (Giuffrida et al. Citation2017). The analysis of low abundant apocarotenoids is becoming increasingly important to gain further insight into their roles in plants and animals. A chemical derivatization based ultra-high performance liquid chromatography-hybrid quadrupole-Orbitrap mass spectrometer (UHPLC-Q-Orbitrap MS) methodology that enhances the MS response signal of plant carotenoid-derived dialdehydes, which are known to be very unstable, has been recently proposed (Mi et al. Citation2020).
As far as carotenoid identification is concerned, UV-Vis spectroscopy, and mass spectrometry with atmospheric pressure chemical ionization (APCI) are frequently used. In particular, positive and negative APCI ionization modes are providing complementary information that can greatly help for example in the identification of carotenoid esters regioisomers; in fact, the negative ionization mode provides a prevalent quasi-molecular ion species in the mass spectrum, whereas in the positive ionization mode a greater compound fragmentation is taking place in the APCI source, thus offering useful information in those analyses especially aimed at the determination of the native carotenoid composition in different matrices. Compiled data on the absorption maxima, absorption coefficients, mass spectra data, circular dichroism data and NMR references of carotenoids are available in the literature (Britton, Liaaen-Jensen, and Pfander Citation2004).
Metabolomics analysis in carotenoid research
More than 750 carotenoids are properly characterized and compiled in the Carotenoid Handbook (Britton, Liaaen-Jensen, and Pfander, Citation2004) and the recently published Carotenoids Database (Yabuzaki Citation2017) compiles information of more than 1000 compounds, many of which not completely characterized. However, only a minor part of all known carotenoids is measured in most experimental studies, as typically the analytical methods are optimized toward a few carotenoid species only (Amorim-Carrilho et al. Citation2014). In this regard, given the vast number of known carotenoids and the fact that novel species are still discovered on a regular basis (Maoka Citation2016) metabolomics may signify an appropriate tool to advance carotenoid research. This omics approach is generally defined as the holistic qualitative and (semi-)quantitative analysis of all metabolites that are present in a biological system, being surveyed at a given time-point under specific physiological conditions (Tugizimana, Piater, and Dubery Citation2013). As these metabolites represent the ultimate end-points of the biological cascade, measuring the metabolome may yield valuable insights about the absolute functional state of the system and unravel intricate biochemical and biological mechanisms (Dettmer and Hammock Citation2004). In carotenoid research, metabolomics may primarily contribute to a better understanding of compositional features (e.g. of dietary sources) and/or intrinsic metabolic processes (e.g. carotenoid metabolization in humans).
Implementation of metabolomics is typically elaborated according to the metabolic profiling and fingerprinting (Shulaev Citation2006). Metabolic profiling is used to measure a large set of known and unknown metabolites, which are closely related to each other through their metabolic pathways or chemical classification. This approach often involves a targeted screening of known compounds. Metabolic fingerprinting is used to map patterns of predominantly unknown metabolites that are descriptive for the system’s metabolic state in relation to the assessed experimental conditions. Although fingerprinting encloses the highest intrinsic potential to advance knowledge, it is most challenging because it starts without a detailed (biochemical) hypothesis.
To perform metabolomics studies, two analytical strategies are predominantly used namely nuclear magnetic resonance (NMR) and mass spectrometry (MS) (Amorim-Carrilho et al. Citation2014). The latter seems most designated for carotenoid profiling or fingerprinting as the sensitivity of this technique outperforms that of NMR, also allowing the detection of minor and low-abundant carotenoid species (Gibbons, O'Gorman, and Brennan Citation2015). Generally, time of flight and orbitrap MS are most frequently employed, both having the ability to perform full-scan and high-resolution measurements, meaning that a virtually unlimited number of compounds can be monitored simultaneously at high mass accuracy (ppm range). The latter is crucial to resolve the hundreds of metabolites that are usually retrieved in the generic extract from the biological matrix under consideration (Nielen et al. Citation2007). Following this, methodologies have been established for carotenoid metabolomics using time of flight (Fraser et al. Citation2007) and orbitrap MS (Bijttebier et al. Citation2013, Citation2014; Van Meulebroek et al. Citation2014). For more details on the various steps of the metabolomics workflow (including data acquisition, data pre-processing, multivariate statistical analysis, metabolite identification, and biological interpretation), we refer to the reviews of Hegeman (Citation2010), Hendriks et al. (Citation2011), Neumann and Böcker (Citation2010), and Sangwan et al. (Citation2015). Up to now, only a few studies have reported on the use of metabolomics to address carotenoid-related research questions (Chu et al. Citation2011; Djuric et al. Citation2009; Lamers et al. Citation2010; Lee and Park Citation2010; Sawada et al. Citation2019). However, it should be remarked that the holistic nature of the claimed omics application often concerns a targeted profiling for a limited number of metabolites. As such, holistic profiling and true metabolic fingerprinting have yet to fully unfold in carotenoid research, holding opportunities in various domains.
Opportunities of metabolomics in carotenoid research
Given their nutritional relevance, there are many efforts to enhance carotenoid levels in crop plants by genetic modification, conventional plant breeding or agricultural practices. Mapping the carotenoid profile of crops and evaluating any alterations in response to agricultural practice or genetic modulation may strongly contribute to this objective. Metabolomics research strategies may also support metabolic engineering in plants, algae and bacteria to use these as “cell factories” for producing specific or novel carotenoids. Indeed, incomplete knowledge about the associated metabolic mechanisms is often the limiting factor for efficient engineering. Hence, metabolomics could complement genomics, transcriptomics, and proteomics toward designing superior biocatalysts in cell factories based on revealed gene-to-metabolite networks (Liu, Zhu, and Jiang Citation2009). Relevant studies in this regard have been performed by Chu et al. (Citation2011), Lamers et al. (Citation2010) and Lee et al. (Citation2014). Alternatively, instead of engineering or influencing carotenogenesis from a fundamental health-related perspective, the objective may be to modulate traits such as flower or fruit color, as determinants of market value (Sawada et al. Citation2019). Eventually, beside the usage of metabolomics in a context of carotenogenesis, investigating the impact of post-production factors such as food storage, transport, and processing may benefit from holistic carotenoid analysis as well (Kotíková et al. Citation2016).
Metabolomics also represents an ideal tool to screen the more common as well as novel (exotic) plants, algae, and other carotenoids sources for their qualitative carotenoid composition/production. This may lead to the discovery of novel carotenoids and also reveal well-characterized carotenoids in specific organisms for which their presence was not assumed or expected. As such, metabolomics provides an expedient strategy to localize specific carotenoids and discover new ones within a wide range of natural carotenoid sources (Takatani et al. Citation2015; Takemura et al. Citation2015). Eventually, metabolomics may also aid in deepening knowledge on carotenoids and their bioactivity in humans by focusing on the biotransformation processes and metabolites that are generated in the human body, which is especially relevant in nutritional and pharmacological research. In this regard, it is generally recognized that ingested carotenoids are extensively metabolized, thereby suggesting that diverse cellular functions may be mediated by the resulting metabolites instead of the intact carotenoids (T. Bohn et al. Citation2015). Biological conversion reactions may comprise enzymatic cleavage, oxidation, reduction, hydrolysis, and interaction with free radicals, which leads to a wide range of chemically diverse metabolites and biological functionalities alike. This concept is comprehensively reviewed by Arathi et al. (Citation2015), presenting a substantial set of discovered metabolites for the major carotenoids and discussing their assumed biological significance. As such, characterization of carotenoid metabolization products is regarded crucial to advance insights on carotenoid bioactivity and real bioavailability. For this purpose, metabolomics could constitute an ideal platform as informative metabolic fingerprints of carotenoids and related metabolites can be generated for various biological tissues and bio fluids (Kopec et al. Citation2010; Manach et al. Citation2009). Eventually, this metabolite-oriented approach also has potential to define food-specific biomarkers or descriptive carotenoid profiles, which are indicators of specific (carotenoid-rich) diet exposure and food consumption (Al-Delaimy et al. Citation2005; Djuric et al. Citation2009; van Kappel et al. Citation2001).
Dietary sources of carotenoids
The major carotenoids in foods and the most studied in relation to human health are the three hydrocarbon carotenes: α-carotene, β-carotene, and lycopene, and the three oxygenated xanthophylls: lutein, zeaxanthin, and β-cryptoxanthin. Currently there is a growing interest in the colorless carotenoids phytoene and phytofluene as they are among the main carotenoids in the diet, they are bioavailable in humans and they may provide health and cosmetic benefits (Meléndez-Martínez et al. Citation2015; Meléndez-Martínez, Mapelli-Brahm, and Stinco Citation2018). Britton and Khachik (Citation2009) suggested a useful criterion to facilitate the categorization of carotenoid content in a particular food, so that the level of a specific carotenoid can be classified into four different concentration groups: low (0–0.1 mg/100 g), moderate (0.1–0.5 mg/100 g), high (0.5–2 mg/100 g) or very high (>2 mg/100 g). At this point it is important to note that the carotenoid levels in food products depend on factors of diverse nature including genotype, climatic conditions of the production area, agronomic factors, cooking, processing and preservation methods (Dias et al. Citation2018; Rodríguez-Amaya Citation2016a; Schweiggert and Carle Citation2017). Since climate change is a major challenge to tackle in agro-food, more studies on its impact on carotenoids are needed. Recently, it has been shown that climate change can have a positive impact on the levels of provitamin A carotenoids of plantains, possibly in relation to changes in the sun’s UV-B index (Dzomeku et al. Citation2020).
Fruits and vegetables
Fruits represent one of the most important sources of carotenoids in the human diet. Commonly cultivated and consumed fruits (including citrus species, mango, papaya, apricots or peaches, among many others) and vegetables (including green vegetables, carrots, red pepper, tomatoes among many others are well-known sources of carotenoids (Britton and Khachik Citation2009; Dias et al. Citation2018; Zhou et al. Citation2020). The study of the carotenoid content of underutilized, non-domesticated and/or exotic plant foods has featured in the last decades and continues being important (Chisté and Mercadante, Citation2012; Diep et al. Citation2020; Turkiewicz et al. Citation2020). As a result, important sources of bioavailable carotenoids including lutein (sastra), zeaxanthin (sastra, corozo, sapote) or lycopene (sarsaparilla, buffaloberry), among others, have been pinpointed in recent years (Delgado-Pelayo and Hornero-Méndez Citation2012; Murillo et al. Citation2010, Citation2013; Riedl et al. Citation2013).
β-Carotene is the most widely distributed and the most important provitamin A carotenoid. Common fruits with high or very high contents of β-carotene are apricots, pumpkin or mango (Britton and Khachik Citation2009; Leong and Oey Citation2012). Orange and yellow vegetables, like carrots and some pepper varieties, and dark green leafy vegetables, like kale, spinach, and lettuce, are rich sources of β-carotene (Beltrán et al. Citation2012; López et al. Citation2014; Reif et al. Citation2013). In Spain, vegetables are higher contributors to the β-carotene dietary intake than fruits, as assessed from the National Survey of dietary intake in Spain 2009–2010 (Beltrán-de-Miguel, Estévez-Santiago, and Olmedilla-Alonso Citation2015). Among vegetables, the higher contributors are: carrot (raw and cooked) 573 µg/day, tomato (fresh, tomato sauce) 299 µg/day; spinach, 129.1 µg/day. Among fruits, the highest contributors are: tangerine, 15.3 µg/day; orange, 12 µg/day; banana 11.2 µg/day (Beltrán-de-Miguel, Estévez-Santiago, and Olmedilla-Alonso Citation2015). There is a huge diversity of fruits growing in tropical areas which contain outstanding amounts of carotenoids (Rodríguez-Amaya Citation2016b). Among these, rich in β-carotene are: buriti (Mauritia vinifera) with 372 μg/g FW, peach palm (Bactrys gasipaes) 55 μg/g FW of β-carotene (De Rosso and Mercadante Citation2007), sapote (Quararibea cordata) or corozo (Aiphanes aculeate) (Murillo et al. Citation2013).
Lycopene is present in high amounts in tomatoes and tomato products, e.g., ketchup and juices, as well as in watermelons and pink grapefruits (Biehler et al. Citation2012; Dias et al. Citation2018; Isabelle et al. Citation2010; Reif et al. Citation2013). Typical contents of lycopene in fresh tomatoes are 2.5–23.3 mg/100 g FW (Shi and Le Maguer Citation2000; Viuda-Martos et al. Citation2014). The bright red color of Rosa sp. fruits is strongly correlated with the content of lycopene and, in some species, with that of rubixanthin (a monocyclic monohydroxyxanthophyll). The amount of lycopene in Rosa mosqueta (392 mg/kg DW) was found to be higher than that of tomato fruits (Hornero-Méndez and Mínguez-Mosquera Citation2000b). The (all-E)-lycopene was the major isomer in Rosa canina (7.4 mg/100 g FW) and Rosa rugosa (7.9 mg/100 g FW), although some Z-isomers were also present, the most important being (13Z)-lycopene (Al-Yafeai, Malarski, and Böhm Citation2018). Lycopene Z-isomers were also tentatively identified in R. rubiginosa, R. multiflora, and R. virginiana (Zhong et al. Citation2016). In sarsaparilla berries (Smilax aspera L.), lycopene is the major carotenoid with 242 μg/g FW (Delgado-Pelayo and Hornero-Méndez 2012).
Gac fruit arils (Momordica cochinchinensis) are exceptionally rich sources of (all-E)-lycopene (164.4 mg/100 g FW), Z-isomers of lycopene and β-carotene, and more important these carotenoids are highly bioaccessible compared to tomato fruits (Müller-Maatsch et al. Citation2016). Lycopene (all E) and the (15Z)-lycopene account for 280.5 and 291.4 μg/g DW in the pericarp and in the pulp, respectively, of fully ripe Pink Guava (Psidium guajava L., “Criolla”), where they accumulate in crystalline chromoplasts (Rojas-Garbanzo et al. Citation2017).
β-Cryptoxanthin is the major carotenoid in mandarins and some orange varieties (Biehler et al. Citation2012; Dias, Camões, and Oliveira Citation2009; Dias et al. Citation2018; Isabelle et al. Citation2010; Stinco et al. Citation2016). β-Cryptoxanthin is important as a provitamin A xanthophyll. Persimmon (Diospyros kaki L.) is one of the most important sources of β-cryptoxanthin, which is present both in skin (283–1254 μg/kg FW) and in pulp (76.5–287 μg/kg FW), strongly dependent on the cultivar (Veberic et al. Citation2010). A significantly higher concentration of β-cryptoxanthin, up to 678 μg/100 g FW, was reported in Chinese persimmon cultivars (C. Zhou et al. Citation2011). β-Cryptoxanthin monopalmitate represents 5% of total carotenoid in the fully ripe Goji berries (Lycium barbarum L.) which correspond to about 2.2 mg/100 g FW (Hempel et al. Citation2017). Similarly, β-cryptoxanthin is mostly esterified and represents 18%–24% of total carotenoids (up to 5.1 mg/100 g FW) in the fruits, and much more in the calyces (3.2 mg/100 g DW) of Physalis alkekengi L. (Wen et al. Citation2017). Esterified β-cryptoxanthin can be also found in sea buckthorn (Hippophae rhamnoides L.) berries (2.1–3.8 mg/100 g DW) (Pop et al. Citation2014) and in loquat (Eriobotrya japonica Lindl.) (54–715.2 μg/100 g FW) (Ferreira de Faria et al. Citation2009). Free and esterified β-cryptoxanthin (including the less common oleate) were found at 42 μg/g FW in sarsaparilla berries (Smilax aspera L.) (Delgado-Pelayo and Hornero-Méndez 2012). Among exotic fruits available on the global market, papaya (Carica papaya L.) (Gayosso-García Sancho, Yahia, and González-Aguilar Citation2011; Schweiggert et al. Citation2011) and yellow passion fruit (Passiflora edulis) (Pertuzatti et al. Citation2015) are good sources of β-cryptoxanthin.
Lutein is present in the human diet mainly through green leafy vegetables, but some fruits and animal products can also contribute to the daily intake. Lutein is the most common xanthophyll in dark green leafy vegetables, e.g. spinach, kale, watercress, broccoli, Brussels sprouts, parsley, and lettuce (Bergquist, Gertsson, and Olsson Citation2006; Biehler et al. Citation2012; Perry, Rasmussen, and Johnson Citation2009, Reif et al. Citation2013).
Important sources of lutein have been described in Panama, including yellow mombin (Spondias mombin, 8.6 μg/g FW), Chinese rose (Pereskia bleo, 8.3 μg/g FW), orange pepper (Capsium annuum, 7.9 μg/g FW), hill cherry (Bunchosia nitida, 7.5 μg/g FW), membrillo (Gustavia superba, 6.7 μg/g FW), purple mombin (Spondias purpurea, 6.3 μg/g FW), okra (Abelmoschus esculentus, 5.2 μg/g FW)among the sources with high levels and squash (Cucurbita maxima, 81.7 μg/g FW), India mustard (Brassica juncea, 53.8 μg/g FW), beet (Beta vulgaris, 53.1 μg/g FW), spinach (Spinacea juncea, 43.7 μg/g FW), watercress (Nasturitum officinale, 42.8 μg/g FW), sastra (Garcinia intermedia, 36.8 μg/g FW), endive (Cichorium endivia, 34.2 μg/g FW) and Romaine lettuce (Lactuca sativa, 21.1 μg/g FW) among the sources with very high levels (Murillo, Meléndez-Martínez, and Portugal, Citation2010).
Zeaxanthin. Even though some relevant sources of zeaxanthin are known (maize, orange and red pepper, eggs), the usual dietary ratio lutein: zeaxanthin is still approximately 5:1 and finding new valuable sources is of great importance. Outstanding sources of zeaxanthin are goji berries (Chinese wolfberries, Lycium barbarum L.) and Chinese lantern (Physalis alkekengi L.) fruits and arils. The common feature of these two species is the high proportion of esterified zeaxanthin with different saturated fatty acids (Weller and Breithaupt Citation2003). Recently, Hempel et al. (Citation2017) characterized in detail the carotenoids in goji berries, finding that zeaxanthin dipalmitate represents 80% of total carotenoids in fully ripe fruits, with 35.7 mg/100 g FW (equivalent of 19.4 mg/100 g FW free zeaxanthin). In the fruits of red Physalis, zeaxanthin was present mostly in esterified form (56%–63% of total carotenoids) and the total zeaxanthin content was up to 13.0 mg/100 g FW. Even though Red Physalis calyces are not edible, they can be used as a valuable zeaxanthin source (10 mg/g DW) for food supplements industry using effective extraction techniques (Huang et al. Citation2016). Sea buckthorn berries are cultivated all over Europe and their popularity has increased due to high content of bioactive molecules (vitamins, unsaturated fatty acids). The amount of zeaxanthin in Romanian sea buckthorn (Hippophae rhamnoides L.) ranged between 19.3–42.4 mg/100 g DW, mostly in esterified form (Pop et al. Citation2014). Unusual zeaxanthin and lutein esters with unsaturated fatty acids (palmitoleic, oleic, linoleic) were reported in sea buckthorn berries (Giuffrida et al. Citation2012). As previously reported the total carotenoid in sea buckthorn is strongly influenced by the cultivar and harvesting time and the esterification of xanthophylls represent a ripeness marker (Andersson et al. Citation2009). Important sources of zeaxanthin have been reported among products consumed in Panama, including canistel (Pouteria campechiana, 19.7 μg/g FW), maize flour (Zea mays, 9.4 μg/g FW), potato (Solanum tuberosum, 7.7 μg/g FW), guanabana toreta (Annona purpurea, 6.8 μg/g FW) among the sources with high levels and sastra (Garcinia intermedia, 84.7 μg/g FW), corozo (Aiphanes aculeata, 79.2 μg/g FW), orange pepper (Capsium annuum, 62 μg/g FW), South American sapote (Quararibea cordata, 46.2 μg/g FW) and membrillo (Gustavia superba, 37.6 μg/g FW) among the sources with very high levels (Murillo, Meléndez-Martínez, and Portugal Citation2010).
Phytoene. Some reported sources with very high levels of this colorless carotene are tomato derivatives (sauce, paste, ketchup), and apricots. Among those with high levels are red pepper (Capsicum anuum, 1.69 mg/100 g FW) , yellow apricots (Prunus armeniaca, 1.35 mg/100 g FW), carrots (Daucus carota, 1.34 mg/100 g FW), white apricots (Prunus armeniaca, 1.26 mg/100 g FW), red grapefruit (Citrus paradisi, 1.25 mg/100 g FW), watermelon (Citrus lanatus, 1.17 mg/100 g FW), orange pepper (Capsicum anuum, 1.01 mg/100 g FW) or tomato (Solanum lycopersicum, 1.00 mg/100 g FW) (Meléndez-Martínez et al. Citation2015).
Phytofluene. Some reported sources with moderate or high levels of this colorless carotene are diverse varieties of apricots (Prunus armeniaca), tomato (Solanum lycopersicum, 0.45 mg/100 g FW) and derivatives, carrots (Daucus carota, 0.57 mg/100 g FW) and red grapefruit (Citrus paradisi, 0.51 mg/100 g FW) (Meléndez-Martínez et al. Citation2015).
Other carotenoids widely distributed in foods but not detected in human tissues or fluids, at least at the levels of the major ones (lutein, zeaxanthin, β-cryptoxanthin, α-carotene, β-carotene, lycopene, phytoene and phytofluene) are the carotenoids with 5,6-epoxide groups violaxanthin, neoxanthin and antheraxanthin. The first two are major carotenoids in photosynthetic tissues, where minor amounts of antheraxanthin can also be found. Therefore, they all are present in green vegetables, although they are also found in non-green tissues of other plant foods, including exotic ones. Other carotenoids, including capsanthin or capsorubin are limited to few species (typically of the Capsicuum genus). Another example is rubixanthin, that is a major xanthophyll in Rosa sp. (Al-Yafeai, Malarski, and Böhm Citation2018; Biehler et al. Citation2012; Delgado-Pelayo, Gallardo-Guerrero, and Hornero-Méndez Citation2016; Dias et al. Citation2018; Hornero-Méndez and Mínguez-Mosquera Citation2000a; Rodríguez-Amaya et al. Citation2008; Rodríguez-Concepcion et al. Citation2018; Zhong et al. Citation2016). More detailed information about distribution and levels of both widely distributed and unusual dietary carotenoids can be found in a comprehensive database (Dias et al. Citation2018) that is further discussed in Intakes in different countries and methods of assessment.
Dietary apocarotenoids that can be found at high or very high levels in some products are bixin (a major component of the colorant annatto) or crocetin, a major carotenoid of the stigmas from Crocus sativus, from which the saffron spice is obtained. On the other hand, minor amounts (sometimes at levels 1000-fold lower relative to the parent carotenoid) of other apocarotenoids derived from β-carotene and lycopene have been detected in dietary sources (Kopec et al. Citation2010; Schaub et al. Citation2017).
Cereals and cereal based products
Lutein, zeaxanthin, β-cryptoxanthin, α- and β-carotene are carotenoids found in cereal grains (Hidalgo, Brandolini, and Pompei Citation2010; Kurilich and Juvik Citation1999). Among cereals, yellow maize has been traditionally considered as the only one with an appreciable carotenoid content; the total carotenoid content in maize grain (11.14 µg/g DW) is up to thirty times higher than in oats, wheat or barley (0.36, 1.50–3.05, and 1.50 µg/g DW) which are found to have very low contents of α + β-carotene and no β-cryptoxanthin (Panfili, Fratianni, and Irano Citation2004). Maize cultivars analyzed by Scott and Eldridge (Citation2005) contained 7.02 µg/g FW of total carotenoids with prevalence of lutein and zeaxanthin (3.30 and 2.09 µg/g FW, respectively) over β-cryptoxanthin, α- and β-carotene (1.04, 0.12, and 0.16 µg/g FW, respectively) while canning and freezing did not reduce their contents markedly.
The variable carotenoid content in maize products is a result of different varieties and/or different processing conditions as was found for canned maize (17.53–27.94 µg/g FW; De Oliveira and Rodríguez-Amaya Citation2007). Additionally, the same authors found that maize flakes and meal had similar total carotenoid contents (15.10–21.28 and 16.37–19.33 µg/g FW, respectively) that were higher than the content in flour (8.25–19.20 µg/g FW). Additional processing decreases the carotenoid content even more: yellow maize tortillas and chips contain 2.13 and 1.42 µg/g DW, respectively (de la Parra, Serna Saldivar, and Liu Citation2007).
Among wheat species, the most widely cultivated is bread wheat (Triticum aestivum), a worldwide staple food. The yellowish color of the endosperm of wheat grains, as well as wheat-based derived products (mainly flour and baked goods) is due to the presence of lutein (Ahmad et al. Citation2015; Lepage and Sims Citation1968; Mellado-Ortega and Hornero-Méndez Citation2015b; Rodríguez-Suárez, Giménez, and Atienza Citation2010). Lutein represents more than 85% of total carotenoid content in most wheat species. Moreover, traces of zeaxanthin, β-cryptoxanthin and β-carotene can also be found. Semolina production is associated with a bright yellow color for pasta manufacturing which has promoted the enhancement of the lutein content in new durum wheat varieties (Ficco et al. Citation2014). Common wheat has been traditionally selected for a white color for bread making (Mares and Campbell Citation2001). Tritordeum, a hybrid cereal obtained from cross-breeding between a wild barley (Hordeum chilense) and wheat, stands out due to its high lutein content in grain (up to 10–12 μg/g DW), which is similar to some einkorn and selected bread wheat cultivars (Alvarez, Martin, and Martin Citation1999; Atienza et al. Citation2007; Mellado-Ortega and Hornero-Méndez Citation2015a, Citation2016; Ziegler et al. Citation2015). Bread wheat, einkorn, spelt, emmer, and tritordeum grains contain an important fraction (>25%) of lutein esters. Lutein is esterified with palmitic and linoleic acids, in the form of monoesters as well as homo- and hetero-diesters (Ahmad et al. Citation2015; Lepage and Sims Citation1968; Mellado-Ortega and Hornero-Méndez, Citation2012, Citation2016, Citation2017; Ziegler et al. Citation2015).
Although wheat has a low carotenoid content, it is used for the preparation of bread and pasta, common foods in diets worldwide. Thus, a high proportion in the diet makes wheat products notable carotenoid sources in human diets. Lutein and zeaxanthin contents in bread and pasta range from 4.5 to 6.3 and 0.08 to 0.12 µg/g DW, respectively (Hidalgo, Brandolini, and Pompei Citation2010). Furthermore, if pasta is prepared with eggs, even higher carotenoid content can be expected: 6.56 µg/g DW for lutein and 1.61 µg/g DW for zeaxanthin with a total carotenoid content of 8.50 µg/g DW (Fratianni et al. Citation2012). Contrary to processed wheat products, processed rice is not a significant carotenoid source in human diets. Processing during preparation of parboiled rice considerably decreases the carotenoid content. Unprocessed parboiled brown rice contains lutein (91–107 ng/g FW) and β-carotene (66–150 ng/g FW) as predominant carotenoids followed by zeaxanthin (14–37 ng/g FW; Lamberts and Delcour Citation2008).
Eggs
The pigmentation of egg yolk is a result of a hen’s ability to absorb carotenoids from the diet and deposit them in eggs. Although hens could use some carotenoids as precursors of vitamin A, the carotenoid profile of egg yolks reflects the carotenoid profile of the diet (Karadas et al. Citation2006). Hence, hens fed a diet based on maize and soybean meal will produce eggs with lutein, zeaxanthin, β-cryptoxanthin and β-carotene; a diet containing 60% of maize resulted in eggs with 14.2, 5.7, 1.3, and 1.4 µg/g of these carotenoids, respectively (González et al. Citation1999). Lutein and zeaxanthin are considered predominant carotenoids in egg yolk available for purchase in stores, but their content is very variable: as an example, while Perry, Rasmussen, and Johnson (Citation2009) reported 9.17 µg/g of lutein and 8.70 µg/g of zeaxanthin, Nimalaratne et al. (Citation2012) found 12.82 and 6.39 µg/g, respectively.
The yolk color is the most important factor influencing consumers’ product acceptance, and desired pigmentation is usually achieved with carotenoid supplementation in diets, especially when low-carotenoid feeds are used. Many sources were evaluated, from both synthetic and natural origin, and the addition of small amounts of red xanthophylls with yellow ones was found to achieve higher pigmentation at a lower cost (Santos-Bocanegra, Ospina-Osorio, and Oviedo-Rondón Citation2004). Thus, it is not surprising that unusual dietary carotenoids including canthaxanthin (3.21–11.56 µg/g), β-apo-8′-carotenoic acid ethyl ester (1.40–11.00 µg/g) or citranaxanthin (2.95–7.11 µg/g) could be found in commercial eggs (Schlatterer and Breithaupt Citation2006). However, it has to be noted that supplementation of canthaxanthin in diets of laying hens has been limited to 8 mg/kg because daily administration of canthaxanthin was associated with crystalline deposits in the retina (Commission implementing regulation (EU) 2015/1486). The use of carotenoids supplements in hens’ nutrition is also dependent on feeding systems: for instance organic and free-range eggs have been reported to contain higher levels of lutein, zeaxanthin and β-cryptoxanthin due to the usage of sources of carotenoids naturally occurring in hens’ diet compared to the eggs from hens housed in barns or cages (Schlatterer and Breithaupt Citation2006).
Eggs are not usually consumed raw, and cooking and processing decrease the carotenoid content. Nimalaratne et al. (Citation2012) reported 8%–15.2% decrease of zeaxanthin and 11.3%–12.8% decrease of canthaxanthin as a result of cooking, with lutein as the most affected carotenoid by processing (22.5% decrease after boiling, 16.7% decrease after microwaving and 19.3% decrease after frying).
Dairy products
Carotenoids contribute significantly to the sensory as well as health properties of dairy products. Carotenoids in cows’ milk are mainly comprised of (all-E)-β-carotene and lutein, zeaxanthin and β-cryptoxanthin can be present to a lesser extent. β-Carotene comprises 90% of total carotenoids present and its concentration is thought to be more variable than that of retinol (Nozière, Graulet, et al. Citation2006). Raw milk, full fat milk, semi-skimmed milk and butter samples have been reported to contain about 6 µg carotenoids and 10 μg retinol per gram of fat. (Hulshof et al. Citation2006). Factors influencing milk yield (i.e., breed, parity, physiological stage, level of dietary intake) control milk β-carotene concentration by concentration/dilution mechanisms, and by efficiency of extraction from plasma (Agabriel et al. Citation2007; Calderón, Chauveau-Duriot, Pradel, et al. Citation2007; Nozière, Grolier, et al. Citation2006). Additionally, levels of β-carotene and lutein present in milk are linked to dietary factors such as the proportion of grazed grass/grass silage in comparison to diets rich in concentrates or maize silage, as carotenoid pigments are particularly high in fresh grass (Martin et al. Citation2004).
In spite of their low percentage in milk, carotenoids (β-carotene and lutein) are involved in the sensorial properties of dairy products. The yellow color of butter and many cheeses is influenced by the β-carotene concentration, whereas high losses of retinol occur during cheese-making (Nozière, Grolier, et al. Citation2006). For Cheddar cheese, O’Callaghan et al. (Citation2017) reported that b* and L* color values were significantly positively and negatively correlated, respectively, with β-carotene content as measured using Hunter L*a*b* values.
However, not all carotenoids present in dairy products originate from milk sources. Smear ripened (also known as washed rind ripened) cheeses, ripened under humid and aerobic conditions; develop a complex growth of halo-tolerant, carotenoid-producing species of bacteria and yeasts. These yeasts (e.g., Kluyveromyces, Debaryomyces, Rhodotorula) and bacteria (e.g., Corynebacterium, Brevibacterium, Arthrobacter, Micrococci) and their associated carotenoids are responsible for the characteristic red/orange/brown colors which contribute to the consumer appeal and also to their intense odor and flavor profile (Galaup et al. Citation2015; Giuffrida et al. Citation2016; Sutthiwong and Dufossé Citation2014). Such bacteria, including those isolated from cheese, have varying abilities to produce pigments and carotenoids; e.g., from 0.14 to 0.6 mg of pigments per g dry biomass produced by Arthrobacter arilaitensis and Brevibacterium linens strains isolated from smear ripened cheeses (Guyomarc'h, Binet, and Dufossé Citation2000; Sutthiwong and Dufossé Citation2014), while Flavobacterium sp. were able to produce 16 mg of zeaxanthin/g dried cellular mass (Dufossé Citation2006). More recently, it was observed that Thermus thermophilus, a carotenoid-producing genus, was present at higher levels within pink cheeses than in control cheeses and the pinking was recreated in cheeses by the reintroduction of a T. thermophilus isolate to a test cheese during the manufacturing process (Quigley et al. Citation2016).
Carotenoids are also added directly to dairy products during manufacture. Traditionally, certain cheeses have been deliberately colored through the addition of annatto (containing the apocarotenoids bixin and norbixin) a colorant used for centuries that is obtained from the outer layer of the seeds of Bixa orellana, a small tropical tree (Meléndez-Martínez Citation2019). Additionally, interest has increased in consumption of dairy products enriched with carotenoids seeking for health benefits. Kubo, Maus, et al. (Citation2013) incorporated a liquid emulsion of lutein during the manufacture of Prato cheese achieving 677 μg of lutein per g of cheese while Jones, Aryana, and Losso (Citation2005) added lutein during Cheddar manufacture to obtain up to 6 mg lutein per 28 gram individual cheese serving. Carotenoids are also added to dairy-based formulated nutritional foods, such as fortification of Infant Milk Formula with lutein for enhanced cognitive and macular health in the neonate. To this end, current research is focusing on ways of protecting the bioavailability of carotenoid emulsions which are subjected to dehydration processes prior to incorporation in formulated foods (Lim et al. Citation2016).
Fish
In the marine environment, carotenoids appear in the animals thanks to their transference and modification throughout the food chain. Carotenoids in the fish diet do not only affect the final carotenoid profile in the fish, but also have an impact on fish color and health (Hisano, Pilecco, and Ferreira de Lara, Citation2016; Kalinowski et al. Citation2005; Pham et al. Citation2014). A large variety of carotenoids has been found in fish, but it is known that fish absorb and accumulate xanthophylls better than carotenes (Schiedt Citation1998). Prominent among these xanthophylls are zeaxanthin, astaxanthin, tunaxanthin and lutein ( and ) and these accumulate in locations including muscle, integuments, liver, eggs, gonads, eyes, brain, intestine, and mouth mucus (Fox Citation1979; Haard Citation1992; Lerfall, Bendiksen, Olsen, and Østerlie Citation2016; Lerfall, Bendiksen, Olsen, Morrice, et al., Citation2016; Tsushima et al. Citation2002). Astaxanthin, canthaxanthin, β-carotene and lutein () have been found in salmon muscle, the main one being astaxanthin, which has been reported in concentrations ranging from 1 to 7 mg/kg (). Carotenoids such as lutein, zeaxanthin, canthaxanthin, β-cryptoxanthin and astaxanthin have been found in the muscle of trout (Pérez-Fernández et al. Citation2017). Concentrations up to 14 and 12 mg/kg of astaxanthin and canthaxanthin, respectively, have been found in fillets of trout specimens which had been fed with those carotenoids (Choubert and Baccaunaud Citation2010). More detailed information about carotenoids in the aquatic ecosystems can be found in some recent reviews (de Carvalho and Caramujo Citation2017; Maoka Citation2011).
Table 1. Carotenoid concentrations in fish (mg/kg).
Livestock
Considering accumulation in the adipose tissue, mammals can be classified in two groups. One group includes animals with a white fat that absorb little or no carotenoids and the other group includes those animals with a yellow fat which can absorb carotenoids. Pig, goat, sheep and rodents belong to the first group, whereas cattle, horses and birds are included in the second group (Álvarez et al. Citation2015; Green and Fascetti Citation2016; Schweigert Citation1998). Thus, for example, the color of bovine fat is due to the presence of β-carotene, the main carotenoid present, and to other pigments such as lutein (Nozière, Graulet, et al. Citation2006; Strachan, Yang, and Dillon Citation1993). Numerous efforts have been made by the scientific community to demonstrate how the diet of animals has a direct impact on their concentration of carotenoids and to determine appropriate feeding approaches to increase this concentration (Adeyemi et al. Citation2016; Álvarez et al. Citation2014; Descalzo et al. Citation2005; Nozière, Grolier, et al. Citation2006). β-Carotene is the main carotenoid present in the serum and adipose tissues of bovines (Chauveau-Duriot et al. Citation2010; Mora et al. Citation2001;Yang et al. Citation2002), while in the plasma of sheep and goats lutein is the main carotenoid (Yang, Larsen, and Tume Citation1992). In fact, these are almost the only carotenoids analyzed in most studies on carotenoids in livestock (). In a study conducted with the liver and muscle of several meat-producing animals, it was estimated that the intake of cow or horse liver provided approximately 0.6 mg of carotenoids per day in an Egyptian adult (Darwish et al. Citation2016).
Table 2. Carotenoid concentrations in livestock.
Alternative sources
The agro-food system is experiencing an important transformation that is urgent in order to provide with sustainable and healthy diets to a growing population (Willett et al., Citation2019). Concepts including sustainability and circular economy must always be associated to food production. Within this scenario, research on “alternative” sources of carotenoids is gaining importance. Among them research on macroalgae (Eismann et al. Citation2020; Xie et al. Citation2020) and microalgae (Dineshkumar and Sen Citation2020; Diprat et al. Citation2020; Rearte et al. Citation2020) carotenoids is well represented in the literature, although fungi, bacteria or insects are other sources that can be further tapped into given their characteristics and production advantages, including diversity, reduced consumption of resources, possibility of optimization of growing conditions for different purposes, etc (Baiano, Citation2020; Mapelli-brahm et al., Citation2020; Ram et al., Citation2020).
Carotenoids in feed
As compared to foods, information about the carotenoid content in feed is scarce. Only high-carotenoid feeds, needed for pigmentation of animal products, have been extensively evaluated. Carotenoid supplementation of diets is thought to increase the oxidative stability of animal products, and the carry-over of carotenoids in the human food chain is advantageous for human health (Golzar Adabi et al. Citation2010; Ma and Lin Citation2010).
Cereals and their products are among the most used feedstuffs in animal nutrition. Despite their high proportions in animal diets, grains contribute little to carotenoid intake since they contain only low amounts of carotenoids (Zhai, Xia, and He Citation2016). Usually, maize has the highest total carotenoid content, followed by barley, wheat, (1.2, 1.5, and 11.1 µg/g DW, respectively; Panfili, Fratianni, and Irano Citation2004). Contents of lutein, zeaxanthin, β-cryptoxanthin and β-carotene varied among commercial maize hybrids, ranging from 6.4 to 16.0, 8.3 to 18.6, 0.9 to 3.1, and 0.6 to 1.5 µg/g, respectively (Kljak and Grbeša Citation2015). Recently, the potential of maize grain as a natural source of carotenoids in poultry diets was recognized, which lead to bio fortification in terms of increased β-cryptoxanthin and β-carotene contents in this feed (up to 2.6 and 4.5 µg/g, respectively) (Liu et al. Citation2012).
Processing of cereal grains decreases carotenoid content (Blandino et al. Citation2017); maize feed flour and germ contained 8.4–11.2 and 6.5 µg/g DW of total xanthophylls compared with 14.4 µg/g DW, for whole grain. With increased intensity of the processing and exposure to air during storage, the decrease in carotenoid content was even higher (Rodríguez-Amaya Citation1997): cracked and steam-flaked maize contained less than 0.4 µg/g DW of provitamin A carotenoids (Pickworth et al. Citation2012). On the other hand, removal of starch during processing results in concentration of carotenoids in the resulting products: total xanthophyll content in maize gluten meal was about seven times higher than in grain (146 µg/g; Moros et al. Citation2002) while in distillers dried grains with soluble (DDGS) it varied from 4.7–33.7 (Robertson et al. Citation2005) to 275.9 µg/g DW (Shin et al. Citation2016).
Oilseeds and their products are not significant sources of carotenoids in animal diets, although they are very important sources of fat, protein and fiber. Rapeseed and its products are the only examples from this feed category that contain higher contents of lutein (µg/g FW)—13.3 in seeds, 14.5 in cake, and 5.7–14.9 in oil (Franke et al. Citation2010). Legume seeds are used in both human and animal nutrition, with the latter including products like hulls, middling and pulp. Lutein is predominant in pea seeds and hulls (6.1–24.5 and 2–25 µg/g, respectively) while seeds contain small amounts of zeaxanthin, β-carotene and violaxanthin as well (0.1–1.3, 0–1.6, and 0.1–1.3 µg/g, respectively; Ashokkumar et al. Citation2015; Marles, Warkentin, and Bett Citation2013). Lupin seed is a better source of carotenoids (53–230 µg/g) with 18.6–24.1, 16.2–135.0, and 12.0–50.4 µg/g of lutein, zeaxanthin and β-carotene, respectively (S. Wang, Errington, and Yap Citation2008).
By-products of the food industry are good carotenoid sources, such as DDGS and gluten meal and feed previously mentioned. Citrus pulp is a widely used fiber source and some samples have been reported to contain up to 30.6% of β-cryptoxanthin and 12% of lutein (Agócs et al. Citation2007) while addition of citrus pulp silage has shown a three-fold increase of β-cryptoxanthin content in cow’s milk (Tanaka et al. Citation2010). Carrot pulp can be used for cattle (4.7 µg/g of lutein, 28.8 µg/g of α-carotene and 58.7 µg/g of β-carotene), and dried carrot meal for poultry (8.0 µg/g of lutein, 39.9 µg/g of α-carotene and 62.8 µg/g of β-carotene; Chen and Tang Citation1998).
In contrast to dry concentrates, feeds and forages used in ruminant nutrition are rich sources of carotenoids; cattle in grass-based production systems generally have carcass fat which is more yellow than that of their concentrate-fed counterparts, due to carotenoids from the lush green forages (Daley et al. Citation2010). Carotenoid contents in forages and roughages are influenced by plant species, stage of growth, harvest and postharvest methods, and season. Significant seasonal shifts occurred in carotenoid content owing to the seasonal nature of plant growth (Elgersma, Søegaard, and Jensen Citation2013, Citation2015). Major carotenoids in forages and roughages are lutein, zeaxanthin and β-carotene while neoxanthin, violaxanthin and antheraxanthin could be found in lower contents (Nozière, Graulet, et al. Citation2006). Forage species differed in carotenoid content in fresh herbage: fresh red clover contained 136 µg of lutein, and 29 µg of β-carotene per g DW (Cardinault et al. Citation2006), while fescue pasture contained 89.3 to 208.9 µg/g DW of β-carotene (Pickworth et al. Citation2012). Forages are often grown in mixture: a mixture of birds’ foot trefoil and timothy contained more β-carotene than a mixture of red clover and timothy or meadow fescue (56.2 vs. 39.1 and 35.6 µg/g DW; Lindqvist, Nadeau, and Jensen Citation2012).
Preserving/processing of fresh forage decreases the carotenoid content; in the process of making silage, haylage or hay, as much as 80% of the carotenoid content can be destroyed (Chauveau-Duriot et al. Citation2005). Grass fresh material consisting of 45% timothy, 45% meadow fescue and a small proportion of couch grass resulted in 29.6 µg β-carotene and 248.2 µg lutein per g of silage DW and 14.3–24.4 g β-carotene and 81.2–141.8 µg lutein per g of haylage DW, depending on the duration of wilting (Müller et al. Citation2007). β-Carotene, like other carotenoids, is sensitive to oxidation, and wilting often reduces its content, especially in sunny weather (Ballet, Robert, and Williams Citation2000). Maize silage, whether whole crop or grain, is considered a poorer source of carotenoids than grass forage (Nozière, Graulet, et al. Citation2006); per g DW, whole crop maize silage contained up to 40.3 µg of β-carotene, 0.8 µg of β-cryptoxanthin and 0.8 µg α-carotene, while high-moisture maize had even lower contents (up to 1.5, 0.8, and 0.4 µg/kg DW, respectively; Gorocica-Buenfil et al. Citation2007; Pickworth et al. Citation2012).
Artificial drying of forages, mainly alfalfa, enables their use in non-ruminant nutrition as well. These dried products are also rich sources of carotenoids: alfalfa protein concentrate contained 1119 µg/g DW, including 697 µg lutein, 247 µg β-carotene, 18 µg zeaxanthin, 69 µg violaxanthin and 46 µg antheraxanthin) (Calderón, Chauveau-Duriot, Pradel, et al., Citation2007). Due to the high carotenoid content, alfalfa dried products are often used as natural sources for pigmentation in poultry.
Although seaweed was historically used in livestock nutrition, there is renewed interest in algae as sources of protein, whether macro algae as seaweed or microalgae. This has also highlighted their role as a source of carotenoids. Algae products as dried biomass and meal are, therefore, also used as sources of pigments for fish and poultry products. Carotenoid profile in algae feed products is highly species dependent; for example, biotechnological production focused on astaxanthin from Haematococcus, β-carotene from Dunaliella and lutein from Scenedesmus (Zaťková et al. Citation2011).
Fish meal is used in animal nutrition, primarily as a protein source. Its carotenoid content, namely astaxanthin, is usually low (3.3–7.2 µg/g DW; García-Romero et al. Citation2014, Kalinowski et al., Citation2005) while marine crab and echinoderm meals contained 12.0 and 6.5 µg/g DW of total carotenoids (García-Romero et al. Citation2014).
In nutrition of specific animals such as poultry and fish, diets are supplemented with synthetic or natural carotenoids to achieve desirable egg yolk and flesh color. European Union Register of Feed Additives (Citation2018) lists eight carotenoids that can be added to concentrate feeds. Although there is still a wide range of synthetic pigments available on the market (Englmaierová, Skřivan, and Bubancová Citation2013; Santos-Bocanegra, Ospina-Osorio, and Oviedo-Rondón Citation2004), consumers have become more concerned about the use of synthetic additives in foods and feeds, and thus interest in natural alternatives has increased. Dried alfalfa and algae products are natural alternatives but other sources, such as petals, plant extracts or by-products, could be used as pigment additives as well. One example is marigold extract (6178 µg total carotenoids/g DW; Karadas et al. Citation2006).
Factors affecting carotenoid levels in plant based food
The carotenoid levels in plant foods depend on factors including genotype, location within the plant, climatic conditions or agronomic factors, including reduced irrigation (increasingly important for the sustainable production of foods), high salinity or electrical conductivity, high leaf to fruit ratio, nitrogen fertilization or even boron stress. Such factors have been dealt with in dedicated original or revision studies in the last years (Borghesi et al. Citation2011; Coyago-Cruz, Corell, Moriana, et al. Citation2017; Coyago-Cruz, Corell, Stinco, et al. Citation2017; Coyago-Cruz et al. Citation2018; Poiroux-Gonord et al. Citation2010; Rodríguez-Amaya et al. Citation2008; Stinco et al. Citation2016).
In this review, attention has been directed to factors more related to the industrial processing and/or marketing stages, namely light, technological treatments and storage conditions. At this point it is important to note that in some studies, technological treatments that are likely to inactivate carotenogenic enzymes are reported to lead to enhanced levels of carotenoids relative to the untreated sample. These results should be interpreted with care as such apparent increases may be indeed due to structural changes leading to enhanced extractability of carotenoids during their analysis or to the use of inappropriate methodologies to evaluate carotenoid retention. Appropriate methodologies to carry out such assessments can be found in the reference guide by Rodríguez-Amaya (Citation2001).
Light
Carotenoids are key in photosynthesis by helping harvesting light and protecting from excess light-derived damage by mechanisms including quenching of excited chlorophyll or singlet oxygen. Another non-photochemical quenching mechanism consists in the interconversion of certain xanthophylls, (usually violaxanthin is enzymatically converted into zeaxanthin via antheraxanthin in the so-called violaxanthin-cycle, which is ubiquitous in higher plants), which also results in energy dissipation (Esteban et al. Citation2015).
Light quantity and quality
Light (duration, intensity and quality of light) is an essential factor that regulates the growth and development of plants (Casal and Yanovsky Citation2005; Chen, Chory, and Fankhauser Citation2004; Folta and Childers Citation2008). The effects of red (R), far-red (FR) and blue (B) lights, as well as their ratios have a significant impact on plant development (Gupta and Dutta Agarwal Citation2017), while the influence of green (G) light has also been studied (Smith, Mcausland, and Murchie Citation2017; Wang and Folta Citation2013). Alterations in light duration, intensity and quality are sensed by photoreceptor proteins that trigger plant responses (Fankhauser and Chory Citation1997; Whitelam and Halliday Citation2007) including the formation of photosynthetic pigments (chlorophylls and carotenoids) (Brazaityté et al. Citation2006; Gupta and Dutta Agarwal Citation2017; Li and Kubota Citation2009; Merzlyak, Melø, and Naqvi Citation2008; Ouzounis, Rosenqvist, and Ottosen Citation2015; Samuolienė et al. Citation2013). Photoreceptors have the ability to sense and respond to light wavelengths in a wide continuous spectral range (Burgie et al. Citation2014). Until today, five photoreceptor families have been identified. The phytochromes mainly absorb at the red and far-red (R: 600–700 nm and FR: 700–750 nm, respectively) part of the light spectrum. Phytochromes have two photo reversible forms, inactive red light absorbing Pr form and active far-red light absorbing Pfr form (M. Chen, Chory, and Fankhauser Citation2004; Lin and Shalitin Citation2003; Quail et al. Citation1995). Blue and ultraviolet-A lights (B: 390–500 nm and UV-A: 315–400 nm, respectively) are perceived by three photoreceptor families; the cryptochromes (Ahmad and Cashmore Citation1993), the phototropins (Christie Citation2007), and a number of members of the Zeitlupe family (Suetsugu and Wada Citation2013). Finally, UV Resistance locus 8 (UVR8) has been identified as the ultraviolet-B (UV-B: 280–315 nm) sensor (Jenkins Citation2014). Moreover, phytochromes and cryptochromes reportedly have activity as sensors of green light (Folta and Maruhnich Citation2007) while them also show synergistic effects (Usami et al. Citation2004). All photoreceptors are involved in photomorphogenesis, and their light signaling pathways are used to fine-tune the plant’s photosynthetic status (de Carbonnel et al. Citation2010).
Artificial lighting has long been practiced in agriculture with the use of light sources such as fluorescent (FL), metal halide, high-pressure sodium (HPS) and incandescent lamps. Nowadays, light-emitting diodes (LEDs) are extensively utilized since they provide several advantages over the traditional light sources. Among other advantages, LEDs have long lifespan, adjustable spectral wavelength, minimal thermal output and high energetic efficiency (Bantis, Smirnakou, et al., Citation2018; Bourget Citation2008; Folta et al. Citation2005).
Artificial lighting
The main aspects studied relating to the use of artificial light are food safety and production, and postharvest storage. Most horticultural products have a limited storage potential of some days to few weeks, due to senescence, weight and firmness loss, over-ripening, decay and physiological disorders. Apart from greenhouse and growth chamber cultivation, artificial lights can be used during storage in order to reduce postharvest losses and to maintain product quality. Especially the abovementioned advantages of LEDs and mainly low heat emission allow these lamps to be employed in many steps of the supply chain, such as precooling, packaging, refrigerated transport and market display.
When applied during greenhouse tomato cultivation, HPS lamps supplemented with FR light led to greater carotenoid concentration of tomato fruits (Hao et al. Citation2016). Hoffmann, Noga, and Hunsche (Citation2016) reported greater carotenoid content of pepper leaves under LEDs compared to FL lights, and also higher values under more B containing treatments, after four weeks of 155 μmol/m2s. Greater carotenoid concentration in chili pepper was found under RB light (Gangadhar et al. Citation2012), while pepper fruits produced more carotenoids under HPS with LED interlighting (lamp placement inside the crop canopy) (X. Guo et al. Citation2016).
Tomato fruits are commonly harvested before maturity and ripening which then ensues during storage or distribution. Storage of tomato fruits in darkness or R LED positively affected lycopene accumulation and red color development, while 7 days of B light application caused a delay in the rise in lycopene concentration, red color formation and ripening (Dhakal and Baek Citation2014). Radiation outside the visible spectra also affects carotenoid content in plants. In a recent study, tomato fruits treated with R light or R supplemented with UV had greater lycopene and β-carotene concentrations compared to darkness or darkness plus UV light (Panjai et al. Citation2017), while R and UV-C also led to greater lycopene values after 4 days of treatment (Liu, Zabaras, et al. Citation2009).
Lutein, neoxanthin, violaxanthin, zeaxanthin, and β-carotene were generally increased in two lettuce cultivars grown in a nursery and under supplementary B containing light treatments (Ouzounis et al. Citation2015). More recent research with lettuce showed that RB LED enhanced carotenoid production compared to W, R, and B LEDs (Amoozgar, Mohammadi, and Sabzalian Citation2017). Low intensity application of W supplemented with B light has also been reported to induce greater carotenoid concentration in the outer leaves of Brussels sprouts, compared to the inner leaves (Hasperue et al. Citation2016). W LED induced considerably greater lutein and β-carotene, and subsequently greater total carotenoid amounts of tartary buckwheat sprouts compared to monochromatic B or R LEDs at 10 days after sowing (Tuan et al. Citation2013). Kopsell, Sams, and Morrow (Citation2017) working with Chinese kale found greater β-carotene, lutein, neoxanthin, violaxanthin, antheraxanthin, and total carotenoids under LED lights compared to fluorescent/incandescent lamps, and increasing percentages of B light positively affected the total xanthophyll cycle pigment pool (zeaxanthin, antheraxanthin, and violaxanthin). Lower carotenoid levels were found in Stevia grown under monochromatic R light, while higher levels were recorded under B and W + R treatments (Simlat et al. Citation2016). Mustard microgreens exhibited enhanced accumulation of important carotenoids (α- and β-carotene, neoxanthin, lutein/zeaxanthin) under basal light (B + R + FR) with supplemental G, Y or O lights compared to only basal light (Brazaityté et al. Citation2015). The same authors found increased violaxanthin and neoxanthin (xanthophyll-cycle carotenoids) in red pak choi under supplemental G light. Craver et al. (Citation2017) working with Brassica microgreens (kohlrabi, mustard, mizuna) found greater carotenoid concentrations under lower light intensities, opposite to what was expected.
Six days radiation with R LED enhanced the nutritional value of Satsuma mandarin by increasing the carotenoid concentration, while B light did not affect carotenoid production (Ma et al. Citation2012). In a later study, lutein and β-cryptoxanthin of Satsuma mandarin fruit flavedo were effectively increased with the combination of R LED and ethylene treatment. The greater expression of a number of genes related to lutein and β-cryptoxanthin production contributed to the results (Ma et al. Citation2015). Irradiation with B light led to greater total carotenoid accumulation in ethephon-de greened mandarin fruit, compared to fruits maintained in darkness. Specifically, a number of key individual carotenoids such as violaxanthin, zeaxanthin, lutein, and β-cryproxanthin were positively affected by B light irradiation (Deng et al. Citation2017). Moreover, Yuan et al. (Citation2017) also found greater carotenoid accumulation of ethephon-de greened mandarin fruits under B light. Satsuma mandarin and Valencia orange juice sacs accumulated more carotenoids under 100 and 50 μmol/m2s of B light, respectively (Zhang et al. Citation2015). Moreover, the authors reported that increases in the genes responsible for β, β-xanthophyll production were consistent with greater concentration of β-cryptoxanthin and violaxanthin in Satsuma mandarin under 100 μmol/m2s of B light, and in Valencia orange under 50 μmol/m2s of B light respectively. In pomegranate, greater carotenoid concentration was exhibited under FL light compared to LEDs (Bantis, Karamanoli, et al. Citation2018).
In summary, there is evidence that controlled environment agriculture with the use of artificial lighting can be implemented for large-scale plant production and postharvest practices. Light affects the quality and shelf life of several horticultural products by enhancing the production and accumulation of phytochemicals such as carotenoids, among others and therefore the nutritional quality of plant-derived products.
UV light as post-harvest treatment
Ultraviolet (UV) irradiation emerged as a possible alternative to currently used postharvest phytosanitary treatments. Research has also highlighted other benefits associated with UV irradiation in postharvest technology including the potential of ultraviolet irradiation in prolonging the shelf-life and maintaining the quality of plant foods (Mditshwa et al. Citation2017). UV light induces stress in plant tissues and stimulates the biosynthesis of defensive secondary metabolites. These inducible effects include the accumulation of antimicrobial compounds (phytoalexin), an increase in the activity of defense enzymes and increased antioxidant compounds such as carotenoids, phenolic compounds or vitamin C (Bravo et al. Citation2012, Citation2013; Cantos et al. Citation2000; Panjai et al. Citation2017).
Artificial UV-irradiation in the field can increase, for instance, the potential health enhancing flavonoids in vegetables. However, UV irradiation is usually avoided in the field because irradiation stress delays plant growth. When irradiating postharvest vegetables, the problem of growth inhibition is avoided. Harvested vegetables take one or more days to deliver from the field to markets and consumers, and consumers normally eat them after several days, while usually storing the vegetables in a refrigerator (Kanazawa et al. Citation2012).
There are three types of UV irradiation, UV-A (400–315 nm), UV-B (315–280 nm) and UV-C (280–100 nm). UV-C irradiation is commonly used in sterilizing food products to control foodborne diseases and, in low dose it may delay ripening, improve firmness and extend the shelf-life of tomatoes. UV-B irradiation is considered as being a useful non-chemical way of maintaining postharvest quality and enhancing antioxidant capacity of tomato fruit (Mditshwa et al. Citation2017; Panjai et al. Citation2017) by improving the content of bioactive compounds mainly carotenoids and polyphenols.
summarizes the key findings of some recent studies involving post-harvest exposure of plant foods to different UV types on carotenoid content. As can be seen, exposure to UV - regardless of the type A, B or C- stimulates the biosynthesis of the major carotenoids in the fruit/vegetable tested, being more effective when applied to unripe fruits/vegetables as they still show room for further ripening and additional carotenoid synthesis. These studies have shown that the contents of capsaicin and lycopene increased in habanero pepper and tomatoes, respectively, during ripening after irradiation. In contrast, UV treatments provoked a decrease in β-carotene (Bravo et al. Citation2012), probably due to an increase in lycopene biosynthesis caused by the activation of enzymes involved in its synthesis pathway (e.g., carotene isomerase) or the inhibition of the enzyme β-cyclase involved in the formation of β-carotene (Van den Berg et al. Citation2000). Moreover, high light intensity could also provoke a photo bleaching phenomenon and lead to the destruction of β-carotene (Young Citation1993). In addition, UV-C irradiation increases the content of cis isomers of lycopene in tomatoes, since light exposure leads to the photo isomerization of all-trans-isomers (Bravo et al. Citation2012; Liaaen-Jensen and Lutnaes Citation2008).
Table 3. Summary of post-harvest UV-treatments and their impact on carotenoids levels in different vegetables.
In summary, the effect of UV light on the content of total carotenoids and individual compounds is dependent on different factors such as type of UV light, intensity of the treatment, ripening stage of the fruit or vegetable during treatment, and time and conditions of storage after treatment. Thus, bearing in mind that fruits/vegetables are often harvested before the full-ripe stage in order to ensure integrity during long distance delivery, post-harvest UV treatment could be a feasible and affordable strategy to enhance carotenoid content during the interval between harvest and delivery to retailers and, eventually, consumers.
Effect of technological processes and storage
Following is an overview on the effects of certain thermal and non-thermal processes and the impact of storage conditions on the carotenoid contents in foods. At this point it is important to note that some technological treatments can affect the overall quality of the product related to carotenoids in different ways. As an example thermal processing can have a negative effect on the carotenoid content and therefore on the color of the product (sensory quality), but a positive effect on their potential bioavailability (nutritional quality) (Mapelli-Brahm et al. Citation2018).
Effect of non-thermal processing of foods on carotenoid content
High-pressure homogenization
High-pressure homogenization (HPH) has been proposed as a valuable technology to promote desirable changes in the physical properties (particle size distribution, pulp sedimentation behavior, turbidity, color, and microstructure) of different plant products (e.g. tomato, banana, pineapple, broccoli, carrots, etc.) in the form of juices, dispersion or emulsions. HPH technology consists of pumping a fluid through a narrow gap valve using high-pressure intensifiers, which greatly increases its velocity, resulting in depressurization with consequent cavitation and high shear stress. Thus, particles, cells, and macromolecules suspended in the fluid are subjected to high mechanical stress, becoming twisted and deformed (Kubo, Augusto, and Cristianini Citation2013). Also, HPH notably reduces the microbial load to levels equivalent to thermal pasteurization (Guan et al. Citation2016). Industrial HPH of raw tomato puree in either one or two-steps, followed by pasteurization at 98 °C for 40 s, did not cause significant losses of lycopene but increased lycopene cis-isomers (considered more bioavailable) in samples treated with 15 + 5 or 10 + 10 MPa two-step HPH (Pérez-Conesa et al. Citation2009). Interestingly, when applied to mango juice preparation, HPH was shown to apparently increase total carotenoid levels by about 12% in samples subjected to 3 passes of HPH at 190 MPa and 60 °C and stored up to 60 days at 4 °C (Guan et al. Citation2016). Higher homogenization pressures (up to 300 MPa and 94 °C) have been reported to cause carotenoid losses of 27% in orange juice, although those changes did not achieve statistical significance (Velázquez-Estrada et al. Citation2013). Another interesting application of HPH regarding carotenoids is the preparation of oil-in-water emulsions. This technique has been shown to be effective for the release of carotenoids from the food matrix thus rendering them more bioaccessible. For instance, in emulsions based on a mix of tomato and red sweet pepper (75% tomato + 25% red sweet pepper) containing 5% or 10% rapeseed oil obtained by high pressure HPH in the range 100–1500 bar, the carotenoid release increased with the pressure applied and the concentration of oil used. So, at 10% oil and 1500 bar lycopene and β-carotene were released by 50% and 72%, respectively. That is around 2-fold the amount yielded at 5% oil and 200 bar (Kirkhus et al. Citation2019).
Other methodologies based on high pressures, such as high-pressure processing (HPP), have been shown to decrease the levels of carotenoids in carrot juice, as a function of the conditions used. Thus HPP at 300 MPa in three cycles was the treatment leading to the highest carotenoids degradation (41%), whereas a much lower degree or degradation (26%) was observed in the samples treated at 600 MPa (Stinco et al. Citation2019).
Ultrasounds processing
Ultrasound is a form of energy generated by sound waves of frequencies from 20 kHz to 10 MHz which is able to produce beneficial modifications in food quality parameters (enhancing for instance viscosity and homogenization). However, due to the critical temperature and pressure conditions which may be linked to the formation of radicals during sonocavitation, the physicochemical effects of ultrasound treatment might also result in quality losses in food products. Off-flavors, metallic taste, and degradation of major and minor compounds may happen (Martínez-Hernández et al. Citation2016). The Ultrasounds processing (USP) processing (frequency 24 kHz, amplitude 100 μm, power 400 W, time 15–60 min, temperature <90 °C) did not significantly change the lycopene content of tomato pulp (Anese et al. Citation2013). Similarly, in carrot juice subjected to USP conditions of 24 kHz at 50, 54, or 58 °C for 0 to 10 min, no significant changes in β-carotene were observed (Pokhrel et al. Citation2017). In contrast, USP conditions of 42 kHz at 30 °C for 10, 20 or 40 min were applied to Cape gooseberry juice, and compared to thermal pasteurization (80 °C/10 min). The contents of the carotenoids β-carotene, α-carotene, β-cryptoxanthin, zeaxanthin and lycopene increased in time-dependent manner upon USP treatment. For instance, after 40 min, carotenoids contents increased by about 2-fold (Ordóñez-Santos, Martínez-Girón, and Arias-Jaramillo Citation2017). Freshly squeezed Chokanan mango juice was treated by paired combinations of sonication (for 15 and 30 min at 25 °C, 40 kHz) and UV-C treatment (for 15 and 30 min at 25 °C). A significant increase in extractability of carotenoids (15%) was observed (Santhirasegaram, Razali, and Somasundram Citation2015).
Pulse electric field processing
Pulse electric field processing (PEF) technology consists of the application of high-voltage pulses (20–80 kV/cm) for short periods of time (ms or µs) to a product placed in a treatment chamber confined between electrodes. PEF treatment may be mild or moderate intensity (MPEF) or high intensity (HPEF) and is generally used for pasteurization of liquid products (as a non-thermal preservation technology) to inactivate microorganisms and enzymes while maintaining the nutritional quality, antioxidant content, and freshness of liquid food (Martínez-Hernández et al. Citation2016). Torregrosa et al. (Citation2005) applied HPEF to orange-carrot juice (80:20, vol/vol) at different field intensities (25, 30, 35, and 40 kV/cm) and different times (from 30 to 340 µs) and maximum temperature of 65 °C. Compared to pasteurized juice (98 °C, 21 s), they reported increases as well as decreases in certain carotenoid levels.
In tomato products, higher apparent lycopene concentrations of 8%–10% were achieved in HPEF-treated (35 kV/cm for 1500 μs in bipolar 4-μs pulses at 100 Hz, <40 °C) tomato juice compared to the untreated juice, likely due to disruption of cell membranes. Greater lycopene levels have been reported at higher pulse frequency and width, and bipolar mode compared to monopolar in HPEF-treated tomato juice. An apparent lycopene content increase of 46% in HPEF-treated tomato juice (35 kV/cm) with bipolar pulses of 7 μs at 250 Hz and temperature below 40 °C was observed (Martínez-Hernández et al. Citation2016).
Effect of drying operations
Among the various drying techniques, air-, freeze-, microwave-and sun-drying are the most thoroughly studied methods. Air-drying provides products that can have an extended shelf life of up to a year, whereas these conventionally dried products are generally of lower quality compared to their fresh counterparts. In the case of freeze-drying, the food materials are dried under vacuum and at very low temperatures, which reduces deterioration and microbiological reactions resulting in higher quality of final products. Microwave-drying offers opportunities to shorten the drying time, thereby improving quality of the final dried product. Preservation of fruits and vegetables through sun-drying, which dates back many centuries, may result in poorer quality and product contamination. Hot-air drying is often used as it needs generally short drying times. However, due to the high temperatures applied, higher losses in certain constituents e.g., antioxidants can be expected (Kamiloglu et al. Citation2016). provides some examples of the impact of drying techniques on carotenoid concentrations.
Table 4. Changes in carotenoids of fruits and vegetables subjected to hot-air/oven (HAO) or freeze-drying (FD).
Effect of thermal processing of foods on carotenoid content
Thermal processing remains the most widely used food preservation approach. The influence of cooking conditions on content of total carotenoids, β-carotene, α-carotene, (13Z)-β-carotene and (9Z)-β-carotene in pumpkins was evaluated by Carvalho et al. (Citation2014). They compared carotenoid content of the raw samples with those cooked in boiled water, steamed pumpkins and samples cooked with added sugar. Pumpkins cooked with steam had apparent higher contents of carotenoids than raw ones or those cooked differently. The only deviation from this pattern was (9Z)-β-carotene which was at a higher level when cooked with added sugar and, in the case of (13Z)-β-carotene, levels were more or less the same when cooked with steam or cooked with added sugar. The contents of α-carotene, β-carotene and total carotenoids were significantly higher when steaming was used in comparison with other techniques of cooking.
In tomatoes, processes such as blanching, pasteurization, cooking, canning, frying, drying, and dehydration reduce lycopene contents and in some cases can favor cis/trans isomerizations. However, these processing operations may also be beneficial, because they may favor the disruption of food matrices (e.g., cell walls and membranes) facilitating the liberation and solubilization of lycopene, often resulting in an increased bioaccessibility of this carotenoid (Capanoglu et al. Citation2008; Martínez-Hernández et al. Citation2016) that could be due, at least to some extent, to the cis/trans isomerizations that heating can cause as cis-lycopene isomers might be more bioavailable than the all-trans (all-E) counterpart in some cases (Honest, Zhang and Zhang Citation2011; Unlu et al. Citation2007).
Pasteurization of diced peach (90 °C for 5 min) led to apparent increased zeaxanthin levels (336%), while decreasing lutein (22%) and β-cryptoxanthin (32%) (Oliveira et al. Citation2016). As regards tomato products, cold-break (60–80 °C for 2–2.5 min), hot-break (90 °C for 5–10 min) or pasteurization conditions (93 °C for 5–10 min or 80 °C for 20 min) did not cause lycopene losses. However, sterilization of tomato at 121 °C for 2 min or 100 °C for 30 min increased lycopene by 20% and 37%, respectively (Martínez-Hernández et al. Citation2016). In contrast, losses of total carotenoids (10%) upon sterilization (117 °C, 23 min) were reported for carrots while carotenoids were not affected by mild (70 °C, 7.5 min) or severe (90 °C, 19.6 min) pasteurization (Vervoort et al. Citation2012).
Effect of storage conditions on carotenoids
Several factors such as time, temperature, light, oxygen, water activity or packaging material may contribute to carotenoid loss during storage. Several studies have reported significant losses of lycopene during storage. For instance, final losses occurred of approximately 60%–70% in tomato juices stored at 4 °C for 3–4 months in polypropylene bottles, or lycopene losses of approximately 65% in canned tomato juice stored at 4, 25, and 37 °C for 3 months. In contrast, a 1-year storage trial evaluating the stability of lycopene and other antioxidants present in commercially available tomato juices during storage at three different temperatures (8, 22, and 37 °C) and packed in either glass bottles or tetra pack revealed that lycopene was very stable regardless of the packaging material used. Only at the end of the storage trial, significant losses (10%–16%) and increase in cis isomerization were observed in juices stored at 37 °C, while no significant losses were observed under refrigeration and room temperature storage (García-Alonso et al. Citation2009)
The effect of different storage conditions on degradation of carotenoids in dehydrated pumpkins were reported by Song et al. (Citation2018). Dehydrated pumpkins (10 g) were packaged in aluminum foil bags. The packaging was carried out in controlled atmosphere conditions (N2). After packaging, the samples were stored in the dark at temperatures of 4 °C, 25 °C and 40 °C. The results indicated that isomerization and oxidation reactions took place. Storage at 4 °C resulted in less changes in comparison with higher storage temperatures with highest loss of (all-E)-β-carotene observed at 40 °C. It was also observed that both β-carotene and α-carotene were less stable than lutein under the same packaging and storage conditions.
Frozen storage also has an impact on carotenoid stability, which depends on factors such as the vegetable/fruit species, the kind of plant tissues or the type of carotenoid studied. In vegetables packed in polyethylene pouches and stored at −27.5 °C for up to 90 days, β-carotene remained unchanged in green beans and broccoli, whereas important losses were observed in the case of peas (70%), spinach (45%) and carrots (41%). Authors explained these decreases as being most likely due to oxidation during frozen storage (Bouzari, Holstege, and Barrett Citation2015). On the other hand, 5,6-epoxide into 5,8-furanoid isomerizations can take place in frozen orange juices, such that carotenoids with 5,8-furanoid groups can be formed from their counterparts with 5,6-epoxide groups. For example, luteoxanthin and auroxanthin can be formed from violaxanthin and mutatoxanthin from antheraxanthin (Giuffrida et al. Citation2019).
Dias, Camões, and Oliveira (Citation2014) tested the stability of carotenoids in minimally processed (crushed) fruits and vegetables during frozen storage at −20 and −70 °C for up to 13 months. In summary, they reported that carotenoids in orange, cherry, peach, apple, and kale were stable (except α -carotene and zeaxanthin in peach) for 13, 9.7, 5.7, 2.5, and 7.5 months, respectively. For these food sample matrices, no significant difference between the freezing/storage at −20 and −70 °C was observed. It should be noted, however, that storage was performed in sealed glass containers and an inert atmosphere (nitrogen), a fact that may explain the better preservation of carotenoids under their experimental conditions due to oxygen removal, thus preventing oxidation.
Behsnilian and Mayer-Miebach (Citation2017) performed a 2-year storage trial of sliced carrots at different freezing temperatures (−15 to −50 °C) in polyamide/polyethylene bags under vacuum. They used the red carrot Nutri Red which contains large amounts of lycopene (84 mg/kg) and β-carotene (37 mg/kg). The contents of α -carotene, β-carotene and lutein remained stable during frozen storage up to two year in the temperature range from −50 to −15 °C, but substantial losses of (all-E)-lycopene (22%) were detected after only three months at −18 °C (typical industrial and domestic freezing temperature), accounting for 57% at the end of the trial. Lycopene was better preserved at −30 and −50 °C with final losses by about 12%. Authors explained the lower stability of lycopene probably due to the presence of dissolved oxygen in the unfrozen phase of carrots together with the fact that lycopene is more prone to undergo oxidation and auto oxidation reactions compared to β-carotene.
Recovery of carotenoids from food processing by-products
Various by-products of food processing are rich sources of carotenoids, including tomato waste for lycopene, carrot, apricot and mango waste for β-carotene and shrimp waste for astaxanthin. However, to make use of these streams, the seasonally obtained by-product must be stored under frozen conditions (Vági et al. Citation2007) or dried and stored at optimal water activity (Lavelli, Kerr, and Sri Harsha Citation2013; Lavelli, Zanoni, and Zaniboni Citation2007) to prevent carotenoid loss.
Moreover, extraction at an industrial scale needs to be economically and environmentally sustainable. In general, the parameters to be optimized are solvent type, presence of co-solvents, solvent to solid ratio, particle size of solids, temperature, pressure and number of cycles.
Solvent extraction
Solid-liquid solvent extraction is the basic and widely used technology for the recovery of carotenoids from food waste. However, it has major disadvantages, namely: i) the large consumption of organic solvents; ii) the high energy required for the solvent-solute mixture separation; iii) the toxicity of some solvents used; iv) low selectivity; and v) possible degradation of thermo-sensitive compounds.
For tomato waste extraction, commonly used solvents are hexane, ethyl acetate and ethanol; solids to solvent ratio varies between 1:125 and 1:10; particle size of solids is in the range of 0.05–0.72 mm, time is between 30 min and 12 h and temperature is in the range 25–70 °C. The amount of carotenoid recovered is up to 6772, 1510, and 16 mg/kg of waste DW for lycopene, β-carotene and lutein, respectively (Strati and Oreopoulou Citation2014).
For shrimp waste extraction at room temperature, acetone, methanol, ethanol, isopropyl alcohol, ethyl acetate, ethyl methyl ketone, petroleum ether, and hexane were studied individually and in mixtures, with solids to solvent ratios between 1: 2 and 1:8, while particle size not specified. The optimized conditions were found to be 60% hexane and 40% isopropyl alcohol, a solid to solvent ratio of 1:5 in each extraction and 3 extractions (duration not specified), resulting in astaxanthin recovery of 43.9 mg/kg of waste (Sachindra and Mahendrakar Citation2005).
Vegetable oils can also be used as solvents. However, extraction of lycopene from dried tomato pomace with a mixture of ethanol and sunflower oil (1:1, vol/vol) only resulted in a recovery of 42.7% of the total lycopene content, compared to traditional solvent extraction (Strati and Oreopoulou Citation2014). Refined sunflower oil was also proposed for carotenoid extraction from shrimp waste, since it was found to give the highest carotenoid yield compared to other vegetable oils studied; however, the yield compared to conventional solvent extraction was not reported (Sachindra, Bhaskar, and Mahendrakar Citation2006).
Ethyl lactate has been suggested as an alternative solvent for carotenoid extraction. It is an environmentally friendly solvent, produced from the fermentation of carbohydrate feedstock, and is completely biodegradable. Strati and Oreopoulou (Citation2011) found that ethyl lactate gave the highest carotenoid yield from tomato waste compared to acetone or ethyl acetate. Maximum yield was achieved by operating with three successive extractions using a dry tomato waste/solvent ratio of 1:10, for 30 min each, at 70 °C.
Ultrasound assisted extraction
The efficiency of ultrasounds in the frequency range of 20 kHz to ∼ 1 MHz to assist solvent extraction is mainly attributed to acoustic cavitation. This phenomenon causes the disintegration of solid materials, i.e., disruption of cell walls, thus increasing the contact between the solvent and the cell content and accelerating mass transfer. The main advantage of UAE is an enhancement of extraction yield, which allows use of lower temperatures and shorter times with respect to conventional solvent extraction. However, use of large volume of solvents is generally necessary. Eh and Teoh (Citation2012) applied an optimized UAE of lycopene from tomatoes and observed that the extraction yield of (all-E)-lycopene increased by 75.9%, compared to optimized conventional methods of extraction and, at the same time, no degradation or isomerization of lycopene occurred. The ultrasonic frequency was 37 kHz and the solvent used was a mixture of n-hexane: ethanol: acetone (2:1:1, vol/vol/vol). The optimized conditions for the above study were 45.6 min (total extraction time), 47.6 °C (extraction temperature) and 74.4:1 vol/wt (ratio of solvent to freeze-dried tomato sample).
Boukroufa, Boutekedjiret, and Chemat (Citation2017) examined recovery of carotenoids from citrus peel waste. Citrus waste represents a significant problem for the food industry on account of large amounts generated annually. In addition, existing technologies for waste recovery are obsolete and new innovative and, if possible, non-thermal technologies are needed. These researchers used high intensity ultrasound to extract various compounds as a green approach. The optimization of ultrasound processing was conducted for the following parameters: US power, temperature and treatment duration. Afterwards the ultrasonic intensities in Wcm2 were recalculated (52, 65, 130, 195, and 208) and related to the concentration of β-carotene (mg/L). β-Carotene increased in linear fashion with increasing ultrasonic intensities and maximum recovery (22.5 mg/L) of β-carotene was observed at intensity of 195 Wcm2. The highest intensity (208 Wcm2) significantly decreased the yield of β-carotene (13.6 mg/L). The higher amount of energy probably caused disruption of cells and further treatment would likely cause even more decrease in the content of β-carotene.
USP in conjunction with Response Surface Methodology has also been applied to optimize the extraction of carotenoids (β-carotene and lutein) from waste products including cantaloupe waste. The study showed that, under the experimental conditions tested, an amplitude of 100%, an extraction time of 10 min, hexane/acetone (80:20 vol/vol) as extraction solvent and solvent-to-solid ratio of 55 mL/g were the best conditions for the extraction (Benmeziane et al. Citation2018).
A response surface methodology approach has been recently used to evaluate the impact of extraction time, temperature and ultrasonic power on the recovery of total carotenoids from gac peel. By using an extraction time of 76 min, 50 °C and 250 W an yield of 269 mg/100 g dry weight was obtained (Chuyen et al. Citation2019).
Microwave-assisted extraction
Microwaves can transfer energy to a solution, which is heated by the mechanisms of dipole rotation and ionic conduction. Heat is generated inside the cells, which causes moisture evaporation and increase in pressure that improves the porosity of the biological matrix and allows better penetration of extracting solvent. MAE enables extraction of target compounds using short time and hence less energy. However, MAE is not suitable for use with heat-sensitive bioactive compounds and it generally requires a large volume of solvent. Solvents with high dielectric constant, such as water and polar solvents can absorb high microwave energy and are usually better solvents than nonpolar ones. In order to extract carotenoids from carrot peels, intermittent microwave radiation at various values of the intermittency ratio, which refers to the fraction of the microwave radiation time to the total processing time in one cycle (1/2, 1/3, and 1/4), was applied. This procedure allowed prolongation of MAE without causing excessive thermal degradation of β-carotene. The solvent consisted of 50% (vol/vol) hexane, 25% (vol/vol) acetone and 25% (vol/vol) ethanol; solvent: solid ratio was 75:1 and microwave power was 300 W, resulting in the extraction of 2764 mg of total carotenoids/kg of waste DW in 7.5 min (Hiranvarachat and Devahastin Citation2014). Response surface methodology was used to study the effect of microwave power, extraction time and oil (flaxseed) to waste ratio on the recovery of carotenoid from carrot juice processing waste. A recovery of ∼75% was obtained using 165 W of microwave power, 9.39 min of extraction time and 8.06:1 g/g of oil to waste ratio (Elik et al. Citation2020).
Enzyme-assisted extraction
For the recovery of carotenoids from plant material, enzymatic treatment with cellulase, pectinase and hemicellulase may be used prior to conventional solvent extraction process to decrease the extraction time and solvent volume in addition to increasing carotenoid yield. Lavecchia and Zuorro (Citation2008) found that pretreatment of tomato waste with cellulase and pectinase led to a 20-fold increase in lycopene extraction. For the recovery of carotenoids from animal sources, treatment with protease leads to increased extraction yield. Babu et al. (Citation2008) found that pretreatment of shrimp heads with trypsin, pepsin and papain enhanced the extraction of astaxanthin, β-carotene, canthaxanthin, lutein, zeaxanthin and crustacyanin.
Pressurized liquid extraction
Pressurized liquid (or solvent) extraction (PLE), also referred to as accelerated solvent extraction (ASE) or pressurized hot-solvent extraction (PHSE) uses organic liquid solvents at temperatures from 50 to 200 °C and pressures from 99 to 148 atm. In the conditions employed, the solvent is always below its critical point and hence it is maintained in the liquid state during the extraction process. As the temperature increases, the solvent dielectric constant decreases, consequently lowering the polarity of the solvent. The advantages of PLE in comparison with conventional solvent extraction are the short extraction time, the possibility to replace apolar organic solvents with “green” solvents and the high yields obtained. However, this method is not suitable for thermolabile compounds. Quan and Turner (Citation2009) applied pressurized hot ethanol containing 0.1% acetic acid and 0.1% butylated hydroxytyrosol for the extraction of astaxanthin from shrimp waste. Optimal conditions were found to be 87 °C, 49 bars and 14 min, while the solids to solvent ratio was not specified. These conditions gave a maximum astaxanthin recovered concentration of 268.5 mg/kg of dry shrimp waste. Mustafa, Mijangos, and Turner (Citation2012) applied pressurized hot ethanol for extraction of carotenoids from carrot peels. Optimized conditions for extraction were solids to solvent ratio of 1:8–1:11, 60 °C, 50 bars, 5 min pre-heating plus 10 min extraction (5 × 2 min). The amounts of α- and β-carotene extracted were 41 and 229 mg/kg of fresh waste, respectively.
High hydrostatic pressure extraction
High hydrostatic pressures, ranging from 100 to 800 MPa or, even more, up to 1000 MPa and moderate temperatures (usually up to 60 °C) can be applied to achieve good extraction yields for carotenoids, with shorter time and lower solvent volume than the conventional solvent extraction. HHPE can favor the mass transfer phenomena leading to increase in diffusivity coefficient.
Strati, Gogou, and Oreopoulou (Citation2015) investigated the use of HHPE in extracting carotenoids, and especially lycopene, from tomato processing waste using a wide range of organic solvents and solvent mixtures. HP assisted solvent extraction was successfully performed at 700 MPa by using lower ratios of solvent to solid (6:1 and 4:1, mL:g) and reduced processing time (10 min), compared to solvent extraction performed at ambient pressure (solvent to solid ratio was 10:1, mL:g and extraction time was 30 min).
Supercritical CO2 extraction
Supercritical CO2 (SC-CO2) extraction technology can achieve comparable carotenoid yield with respect to the traditional solvent extraction. Besides, CO2 is a nontoxic, non-flammable, nonpolluting and cheap substance and no solvent traces remain in the extract. This aspect makes CO2 preferable to organic solvents. The drawbacks are the greater costs of investment linked to the supercritical technology. For tomato processing by products (including dried skins and seeds), SC-CO2 conditions applied were: pressure: 300–460 bar, CO2 flow rate: 0.792–4 kg/h, temperature: 55–100 °C, time: 1.5–6.5 h, particle size: 0.3–3 mm, co-solvent: ethanol 0%–16% (Strati and Oreopoulou Citation2014). Sabio et al. (Citation2003) reported that 80% of the lycopene and 88% of the β-carotene contents of tomato by-products with average particle size of 0.345 mm was obtained by SC-CO2 extraction, using CO2 flow rate 0.792 kg/h, at 300 bar and 80 °C. Besides the yield, the conditions applied affect the selectivity and hence result in remarkable differences in the composition of the extracts, which may influence their stability. Indeed, yield of lycopene was increased up to 90.1% by increasing the pressure of SC-CO2 to 460 bar at 80 °C. Conversely, the maximum tocopherol content was achieved by SC-CO2 at 300 bar and 80 °C (Vági et al. Citation2007). For dried shrimp waste with particle size ˂ 2 mm, maximum carotenoid recovery, equal to 57%, was achieved by SC-CO2 extraction using 10% ethanol co-solvent, CO2 flow-rate of 3.4–4.8 L/min at 320 bar and 45 °C (Félix-Valenzuela et al. Citation2001). For apricot by-products with particle size in the range 0.106 and 0.300, pressure between 30 and 50 MPa, temperature between 40 and 60 °C, CO2 flow rate of 1.66–3.33 × 10−8 m3/s and extraction time between 4000 and 7000 s, the yield of β-carotene was in the range 50–60 g/kg of dry apricot by-products (Döker et al. Citation2004). Mango peel waste was subjected to two sequential extraction steps: SC-CO2, followed by PLE ethanol applied to the residue of the first stage. Both extractions were carried out at 30 MPa and 40 °C and the duration was 450 min for SC-CO2 and 350 min for PLE. (Garcia-Mendoza et al. Citation2015). In addition to food products, by-products and/or waste, research on innovative extraction methodologies for the obtaining of carotenoid-rich fractions from alternative sources, mainly microbial, are gaining importance (Sarkar et al. Citation2020; Schüler et al. Citation2020; Zhang et al. Citation2020).
Carotenoid databases
The importance of food and its composition in relation to human health has underpinned the long-standing and wide-ranging interest in food composition among scientists, manufacturers, regulators and consumers. Additionally, its variability and analytical difficulty (e.g., specific equipment, time and cost) has led different countries to create and update Food Composition Tables as resources providing detailed information on the nutritional composition of foods. These tables are then utilized in different countries to compare the nutrient intakes by their populations to establish nutrient requirements; produce accurate labels; conduct epidemiological studies on the relationship between nutrient intake and disease; promote the choice of plant and animal foods with good nutritional profiles; guide nutrition education programs; serve as a base for agricultural/animal breeding programs; formulate nutritionally balanced institutional and therapeutic diets; inform consumers about good food choices; design specific diets.
The advent of the increasing importance of quality and traceability issues in all areas, and the simultaneous development of informatics, has enabled the advance to relatively complex and specific data management software (e.g., FAO/INFOODS compilation tool, www.fao.org/infoods/infoods/software-tools/pt/; FoodCASE®, www.playground.foodcase-services.com). These resources can hold large amounts of data and facilitate their access and manipulation. Efforts are being made to increase the availability of data as well as the traceability and comparability of food composition databases through harmonization/standardization. Many European Food Composition Databases have become available online, through the FoodEXplorer interface, a move influenced by EuroFIR within Europe (www.eurofir.org/food-information/foodexplorer). Only with high-quality and validated composition data it is possible to meet the challenges of food quality, nutrition and public health and in the end for consumers to make healthier dietary choices. In this regard, the standard BS EN 16104:2012 (Citation2013) was developed relative to food data, structure and interchange format, for compiling and disseminating food composition data that are comparable and unambiguous with respect to the identity and description of foods, components and compositional values.
Generally, these databases contain data on composition of foods frequently eaten by a large part of the country population that contribute significantly to the intake of nutrients and energy. Sometimes foods of importance for specific population groups are included. However, databases that report the composition of foods with respect to constituents that are not considered as nutrients are rarer, either because their importance for human health has only recently been established or there is still insufficient evidence of positive effects, but also may require more complex analytical methods. Carotenoids include a group of compounds, which are effectively nutrients, i.e., provitamin A carotenoids. Other carotenoids, although not considered as nutrients, may eventually be shown to be of benefit to human health, as for example the well-established association of lutein with eye health, and still others for which further studies are needed.
In the vast majority of food composition databases, carotenoids are included with fat soluble vitamins, as contributors to vitamin A. However, individual carotenoids are rarely presented, or else only for a few foods, due to lack of available data ().
Table 5. Carotenoids in food composition tables (http://www.eurofir.org/food-information/foodexplorer/).
In food composition databases, the important issues of bioaccessibility and/or bioavailability of nutrients are not addressed as these are affected by several factors including the type of carotenoid, the food matrix in which the carotenoid is incorporated, and host-related factors among others (Rodríguez-Concepcion et al. Citation2018). However, in some databases, biological activity is taken into account specifically for the contribution of provitamin A carotenoids (retinol equivalents and retinol activity equivalents) ().
For all the databases shown in , retinol equivalents or retinol activity equivalents (or both) are presented. However, the presence of two options in recent updated databases is evidence of a not well established and validated index for carotenoid activity as vitamin A. Despite the fact that β-carotene (or β-carotene equivalents) are shown in almost all food composition tables, the vast majority lack data, for total provitamin A carotenoids, thus resulting in sub-estimation of β-carotene equivalents, as well as data for lycopene, lutein and zeaxanthin. Only the Swiss database refers to recommended daily intakes for carotenoids, and states that the daily requirement of β-carotene is estimated to be 2–4 mg and the tolerable upper intake level is considered to be 10 mg per day.
Some food composition databases also include supplements for specific analytes (e.g., amino acids, fatty acids, vitamin vitamers, specific bioactive compounds). Data on the bioactive compounds in foods are extensively gathered in three databases, BioActive Substances in food Information Systems, eBASIS (EuroFIR), flavonoid database (USDA) and polyphenols, Phenol-Explorer (INRA). The eBASIS was the most recently updated (2017) and it is unique in the inclusion of data for plant-based bioactive compounds with putative health benefits. This database includes 70 food plants with values for carotenoids, anteraxanthin, cryptoxanthin (presumably β-cryptoxanthin), lutein, lycopene, neoxanthin, violaxanthin, and zeaxanthin, corresponding to 852 data points.
Countries such as Spain, Austria, Switzerland, Brazil and USA have developed carotenoid tables (Beltrán et al. Citation2012; Holden et al. Citation1999; Murkovic et al. Citation2000; O’Neill et al. Citation2001; Reif et al. Citation2013; Rodríguez-Amaya, Kimura, and Amaya-Farfan Citation2008), and others such as Luxembourg, Portugal and Costa Rica published studies on the evaluation of carotenoid contents of traditional foods (Biehler et al. Citation2012; Dias, Camões, and Oliveira Citation2009; Monge-Rojas and Campos Citation2011). A recent initiative of Ibero-American countries, IBERCAROT network (http://www.cyted.org/es/ibercarot), funded by the Ibero-American Program for Science, Technology and Development (CYTED, www.cyted.org) gathered, from peer-reviewed literature, about 660 different food items, fruits and vegetables produced in Ibero-American countries, corresponding to 191 species, 42 carotenoids and 2800 data points (Dias et al. Citation2018).
Taking into account the factors influencing the carotenoid content and profile of foods, beyond the parameters related to analytical method, sample data documentation is especially important and should be addressed in food carotenoid databases, including biodiversity (varieties, cultivars, accessions, breeds), cultivation method, production location, harvest season, luminosity conditions, storage, processing and preparation state, color, maturity stage, part/source, fortification/enrichment level, wild/domesticated, edible portion/waste. For manufacturing food products, although their formulations and quality requirements may be similar, the ingredients dissimilarity may be a source of variation. Compositional variation can be expected among comparable manufactured foods. The two food groups that mostly contribute to the ingestion of carotenoids, fruits and vegetables, are particularly sensitive to these variations, even in case of nutrients. This emphasizes the importance of data documentation for data quality assurance and comparability of results/studies made from these data composition. This is particularly important nowadays owing to globalization and the ease of transportation. Accurate data are of great importance to support research on potential positive health benefits, to improve the estimations of intake, to establish dose activity relationships of biological effects, and to recommend daily intakes.
As direct analysis of foods is the preferred way of obtaining food component data and as analytical methods are already sufficiently developed to achieve results with low uncertainties but still involving high costs and time, databases generally present average or median values. Therefore, care should be taken when designing sampling plans to ensure the representativeness of the food products for which the property is intended to be measured. If the objective is to know the intake of a certain food component by the population, composite samples in which different sub-samples are weighted according to relative shares of the retail market, are required. Taking into account the relationship between foods containing carotenoids and the possible positive effects on human health, and on the other hand, the need to obtain high quality data to achieve results with the level of significance appropriate to the conclusions, cooperation and sharing among food carotenoids data producers will save resources and enable the prioritization of investment in obtaining missing analytical data.
In addition to their important current role in science, carotenoid food databases will also constitute a historical archive for future generations, to track future changes associated with climate and cultivation.
Intakes in different countries and methods of assessment
In general, the main contributors to carotenoid dietary intake are fruit and vegetables, and to a lesser extent animal sources (e.g., egg yolk) and food additives (colorants, E160a-carotenes, E160d-lycopene, E160c-paprika, capsanthin, capsorubin, E161b-lutein, E161g-canthaxanthin). Carotenoids are also included in the formulation of food supplements and in fortified foods (mainly multivitamin drinks and instant chocolate drinks). It has been estimated that a balanced and varied diet supplies about 50 different carotenoids that may be absorbed and metabolized (Khachik et al. Citation1997; Maiani et al. Citation2009), but only eight major carotenoids (including different geometrical isomers in some cases) are usually present in blood (β-carotene, α-carotene, β-cryptoxanthin, lutein, zeaxanthin, lycopene, phytoene and phytofluene) (Meléndez-Martínez et al. Citation2013) which are considered to reflect short-term dietary intake. Serum carotenoid concentration is widely accepted as a good biomarker of fruit and vegetable intake (Souverein et al. Citation2015). The main interest in carotenoids in the context of diet and health/disease has focused on the six major carotenoids in blood; however, there is an increasing knowledge on the role of other carotenoids, such as astaxanthin, neoxanthin, violaxanthin, phytoene, phytofluene, among others, regarding their potential biological activities in the human body. The individual carotenoid intakes in the European diet have been assessed in few studies, although there are many in which only β-carotene is assessed. Data on phytoene and phytofluene intakes have been reported for the population of Luxemburg (Biehler et al. Citation2012) and on neoxanthin and violaxanthin for the Brazilian population (Vargas-Murga et al. Citation2016).
Dietary carotenoid intake shows a high variability both within and between subjects in different populations and seasonal variations in individual carotenoid intake have been reported in some European countries while not in others (Almeida, Serra, and Dias Citation2017; Granado et al. Citation1996; Maiani et al. Citation2009; O’Neill et al. Citation2001; Scott et al. Citation1996; Rodríguez-Concepcion et al. Citation2018). Variation in carotenoid intakes over time has been assessed in some countries (e.g., Denmark, Spain) (Beltrán-de-Miguel, Estévez-Santiago, and Olmedilla-Alonso Citation2015; Estévez-Santiago, Beltrán-de-Miguel, and Olmedilla-Alonso Citation2016; Granado, Blázquez, and Olmedilla Citation2007; Leth, Jakobsen, and Andersen Citation2000) and can be partly explained by variations in fruit and vegetables consumption (e.g., in UK, low in the North), socioeconomic status, cultural factors, and in addition changes in marketing that could contribute to changing life style (Conklin et al. Citation2014).
Data available from studies conducted in different countries are compiled in . There are many data on dietary intake of individual major carotenoids in groups of subjects but data from representative sample populations in different countries are scarce, (e.g., from total diet in USA, Spain, Portugal) or from the overall fruit and vegetable intake (e.g., Brazil). However, information on the intakes of individual carotenoids in European countries is limited (e.g., Spain, Portugal). An interesting five countries study (O’Neill et al. Citation2001) reported comparative inter-country data on individual dietary carotenoids from comparably-aged subjects using a common food frequency questionnaire and the same carotenoid database. However, although it was assumed that those subjects consumed the typical food intake pattern of their respective countries, they were not a representative sample of the overall population of each country, because the sampling was not representative regarding selection of people, places and size. In general, comparisons of results across different studies should be done with caution since several variables, such as sample size and methodology, often differ among studies. Differences in the databases of carotenoid composition in foods and the types of dietary questionnaires employed could also partly explain the very different intake levels found in the literature. To help overcome these types of limitations, for comparisons among populations, the estimation of the relative contribution of each carotenoid to the total carotenoid intake has been suggested (Maiani et al. Citation2009).
Table 6. Carotenoids intakes in different countries.
In Spain, 89%, 68%, and 97.1% of the β-carotene, lutein + zeaxanthin and lycopene dietary intake, respectively, comes from fruit and vegetable consumption (Beltrán-de-Miguel, Estévez-Santiago, and Olmedilla-Alonso Citation2015; Estévez-Santiago, Beltrán-de-Miguel, and Olmedilla-Alonso Citation2016). In Portugal, 15%, 20%, 34%, 27%, 37%, 24% of α-carotene, β-carotene, β-cryptoxanthin, lutein, lycopene and zeaxanthin, respectively, comes from fruits and vegetables groups classified accordingly to FoodEx2 (composite dishes and fruit juices containing these items were not classified as fruits and vegetables) (EFSA Citation2015). These data highlight the importance for harmonized methodologies to make comparisons, because even though the total individual carotenoid intake may be similar in two countries, the use of different food classification systems could eventually result in dissimilarities in carotenoid intake data between the two countries.
Evaluation of carotenoid intake to help defining recommendations to improve population health depends on updated and quality data on food carotenoid composition, on eating habits, food consumption, and on harmonized methods of assessment. Carotenoid intake assessment is complicated due to high variability in carotenoid intake, within- and between-subjects, and to the inaccuracies associated with dietary assessment methods and to the inconsistencies in food composition tables and databases (Maiani et al. Citation2009). Dietary assessment, as the other approaches to assess nutritional status (anthropometry, biomarkers and clinical), has advantages and limitations when applied in individual or population studies (Patterson and Pietinen Citation2006).
Information related to population food consumption could be obtained directly through National Food Surveys conducted accordingly to the latest recommendations (EFSA Citation2014) and involves gathering information on food consumption for two nonconsecutive days of representative population groups. The methodology for data collection from infants and children is the 24-hour food diary, followed by a computer assisted personal or telephone interview. This process involves special attention in the planning phase to ensure representative sampling. The sample size is particularly important, especially in countries with a great variety of diet types due to regional or socio-economic differences. Ideally, food consumption information should include food supplements because carotenoids are components of some of them. Food descriptors are also a very important point when comparisons among countries are done. Other tools such as portion sizes, standard recipes, retention factors and yields are essential to collect accurate food consumption data. Less frequently eaten foods and the usual consumption of food supplements should be assessed in the surveys through short, non-quantitative food propensity questionnaires. Assessment of the prevalence of under- and over-reporting of dietary intakes should be performed as a measure of data quality. Updated methodology recommendations are essential to conduct harmonized, high-quality surveys that result in comparable data among European countries.
As this methodology is expensive and time consuming, intake assessment is frequently made indirectly, taking into account as a unit of study the household and not the individual. Usually these household studies are grouped: a) food balance sheets, b) budget and family expenses surveys, c) specific surveys of household consumption (Martin-Moreno and Gorgojo Citation2007). Of these approaches, food balance sheets are frequently used, where information is presented per capita and obtained by dividing the total annual amounts of each food by the population of the country in the year studied (kg/capita/year or g/person/day), assuming a constant consumption throughout the year.
The conversion of the mean quantity of food to the quantity of carotenoids consumed each day by each person is usually done using food composition databases. At this stage food classification is very important and codification systems such as LanguaL or FoodEx2 should be used. Because the carotenoid profile and content in foods are influenced by various factors, including geographical location and climate, seasonality, growing conditions, etc. (Maiani et al. Citation2009), it is preferable to use food composition databases from the country where the assessment of carotenoid intake is being carried out, provided that they meet minimum quality criteria. In Europe, Food Composition Tables built according to the European standards are available in the EuroFIR platform using the FoodExplorer tool. The main limitation of the food composition tables is the lack of individualized data on carotenoid content.
Based on National Food Surveys, Total Diet Studies (TDS) are a Public Health tool used to determine a population's dietary exposure to harmful chemicals (e.g., heavy metals, mycotoxins, pesticide residues) or beneficial (e.g., nutrients, bioactive components) by analyzing foods as consumed. TDS for a detailed exposure assessment involve analysis of aggregate samples, often also considering different regions and seasons, a relatively detailed approach to samples composed of individual foods (e.g., oranges) or of related types (e.g., citrus fruits and citrus juices). The advantages of an evaluation through TDS are: the ability to determine the approximate daily intake of contaminants/nutrients through a relatively low number of samples, the analyses performed on composite samples instead of individual foods, a more realistic approach to the assessment of exposure to contaminants/nutritional consumption, analyses in foods as consumed (part edible, cooked, prepared, tempered, etc.) and the entire diet is covered. The disadvantages of TDS are: the dilution effect inherent in the use of composite samples, foods with high levels of the substance of interest may not be identified in a composite sample, impossibility/difficulty of composite samples to represent food patterns of specific population groups (e.g., different ages, sex, and ethnic groups).
Because carotenoids constitute a large group of compounds, dietary intake of carotenoids can be analyzed from several perspectives, carotenoids with provitamin A activity, carotenoids without provitamin A activity or each carotenoid individually. The compounds with provitamin A activity could be very important for specific population groups, as for example vegans. Data on individual carotenoids intakes (α-carotene, β-carotene, β-cryptoxanthin, lutein, lycopene, zeaxanthin, phytoene and phytofluene) are of great interest due to their association with lower prevalence of some chronic diseases (e.g., lutein and zeaxanthin with cataracts and age-related macular degeneration, β-cryptoxanthin with bone mass, lycopene with prostate cancer, or the colorless UV-ligh absorbing carotenes phytoene and phytofluene with photoprotecion) (Eggersdorfer and Wyss Citation2018; Meléndez-Martínez Citation2019; Rodríguez-Concepcion et al. Citation2018). In the last nutrition and health survey in the USA, it was shown that carotenoid interactions had different effects in relation to causes of mortality (Shardell et al. Citation2011), however to obtain the strongest evidence of the relationship between carotenoid intakes and health more accurate and harmonized data about food composition and consumption data are needed.
Carotenoids as colorants
Colorants, as one of the groups of food additives by legislation (Regulation (EC) No. 1333/2008), are substances that add or restore color in a food. Colorants can be divided into three categories: natural (extracted from plant, animal or mineral sources), nature-identical (man-made compounds which are also found in nature), and synthetic (manufactured, not found in nature) (Oplatowska-Stachowiak and Elliott Citation2017). Even though synthetic colorants have higher stability and a larger pallet of colors, there is a growing consumer concern with regard to their potential risk to human health (Oplatowska-Stachowiak and Elliott Citation2017). In fact, their consumption has been purported to be associated with adverse health effects such as hyperactivity, irritability, sleep disorders, attention problems, and aggressiveness in children (Arnold, Lofthouse, and Hurt Citation2012; Masone and Chanforan Citation2015). Therefore, the food industry is under pressure to remove the commonly used synthetic colorants from foods and to replace with natural compounds. (Dawson Citation2008; Náthia-Neves and Meireles Citation2018). Nearly all carotenoids absorb maximally light in the visible region of 400–500 nm range, presenting a range of colors between pale yellow (ζ-carotene), intense yellow (diverse xanthophylls), orange (β-carotene) or red (lycopene) (Meléndez-Martínez et al. Citation2007). In fact, the range of colors of carotenoids can be extended to blue (e.g. α-crustacyanin and linkiacyanin) or purple (violet carotenoprotein) by establishing complexes with proteins and forming carotenoproteins (Delgado-Vargas, Jiménez, and Paredes-López Citation2000; Pereira, Valentão, and Andrade Citation2014). By the Regulation (EC) No. 1333/2008, a food additive may be included for the functional class of colors only if it serves one of the following purposes: (a) restoring the original appearance of food of which the color has been affected by processing, storage, packaging and distribution, whereby visual acceptability may have been impaired; (b) making food more visually appealing; (c) giving color to food otherwise colorless.
All food additives are subject to a safety evaluation by the European Food Safety Authority (EFSA) before they are permitted for use in the European Union by the European Commission. Food additives are kept under continuous observation and are reevaluated by EFSA (EFSA ANS Panel Citation2016). A program for the reevaluation of food additives that were already permitted in the EU before 20 January 2009 has been set up under the Regulation (EU) No. 257/2010. Food additives (including colorants) to be reevaluated by EFSA have been previously assessed for their safety by the Scientific Committee on Food (SCF).
The maximum levels for colors are set out in legislation and mostly shall apply to quantum satis or the quantities of coloring contained in the coloring preparation (Regulation (EC) No. 1333/2008). The European Food Safety Authority (EFSA) has made recommendations defining safe daily intakes of carotenoids which are much higher than the normal carotenoid intakes in different countries discussed earlier. As an example, the acceptable daily intake (ADI) for lutein from Tagetes erecta used as an additive is 1 mg/kg body weight per day or that of lycopene as additive is 0.5 mg/kg body weight per day (Meléndez-Martínez Citation2019).
The specifications of food additives relating to origin, purity criteria and any other necessary information, is adopted when the food additive is included in the Community lists (Commission Regulation (EU) No. 231/2012). The required inclusion of these substances on food labels is correlated with their classification in accordance with the International Numbering System (INS): a peculiar identification code is assigned to all EU approved additives, consisting of the letter “E” followed by 3 or 4 digits. However, each additive may be also indicated on the label with their chemical name. Currently carotenoids as colorants are divided into two categories with special E code: carotenes (E160) and xanthophylls (E161) (Laganà et al. Citation2017).
Source of carotenoids currently used as colorants in the EU
β-Carotene colorant currently permitted for use can be obtained through chemical synthesis or using three different natural sources, namely, extraction from yellow/orange vegetable matrices (e.g. Elaeis guineensis and Mauritia vinífera) or produced by fermentation and bioprocess engineering methods using algae (Dunaliella salina) and fungi (Blakeslea trispora) (Pereira, Valentão, and Andrade Citation2014). Since 1950, synthetic β-carotene has been produced using a rapid method that applies β-ionone as precursor, and still represents the majority of carotene commercialized in the world (Ribeiro, Barreto, and Coelho Citation2011). Lycopene can be produced synthetically or extracted from tomatoes which are preferably produced in greenhouses for the control of environmental conditions, and tomato processing by-products (pulp and skin) (Ciriminna et al. Citation2016; Pereira, Valentão, and Andrade Citation2014). β-Apo-8-carotenal, canthaxanthin and astaxanthin have been present in the market of colorants mostly through chemically synthesis. Recent studies have reported that Haloferax alexandrines are one of the most promising microorganisms for the extraction of natural canthaxanthin (Chandi and Gill Citation2011). Astaxanthin was firstly synthesized in 1975 and even though its $220 million global market is dominated by the synthetic version (95%), consumer demand for natural colorants has increased the extraction of this xanthophyll from natural sources, mainly from H. pluvialis and to lesser extent from shrimps (Pandalus borealis) (Ambati et al. Citation2014).
Patents on carotenoids in relation to foods and feeds
The World Intellectual Property Organization (WIPO) and the European Patent Office (EPO) allow the search of their registered patents using Patentscope and Espacenet online search engines. Using “carotenoid” as a keyword in the search engines, 19,926 results were found in WIPO and 3759 in EPO. Also, when using “xanthophyll” as a keyword, 50 matches were found in WIPO and 5 in EPO.
To facilitate the presentation of the large number of identified patents, a classification is presented in terms of carotenoid source; isolation techniques; formulation of carotenoids; and food and feed applications of carotenoids ().
Patents related to carotenoid sources and extraction techniques
In WIPO and EPO databases 7664 and 199 patents, respectively, are found when “carotenoid + extraction” keywords were used. Applied extraction techniques for carotenoids used in the food industry cover a wide range of starting materials like fruits and vegetables, other terrestrial plant materials, algae, microorganisms, sludge and waste material from plants and shellfish.
There are many patents describing isolation of lycopene from tomato peel (Basim and Nice Citation2017) and tomato (Mun et al. Citation2012; Xingjun et al. Citation2011; Yoo Citation2006), offering simple methods that protect lycopene from degradation or to make it water soluble. Lycopene from plants can be efficiently extracted by treating the plant material with a mixture of alcohols of C1 to C4. After filtration, the cake is treated with talc and nonpolar organic solvent having a dielectric constant of 6.0 or less (Kim et al. Citation2016). Astaxanthin and lycopene from genetically-engineered tomato fruit were efficiently extracted using supercritical CO2 (He and Huang Citation2017), while astaxanthin fatty acid esters have been isolated from a plastid-genome modified plant (Misawa, Harada, and Maoka Citation2014).
Green mass of plant material can be used for producing oil carotenoid concentrate, where the extraction is performed with vegetable oil (0.5:1–2:1 ratio of oil: raw material), after the washing, drying and milling of the raw material. The concentrate obtained can be used as food additive, biological additive in cosmetics and in medicine and veterinary applications (Postoienko et al. Citation2004). Oleoresin is extracted from wild or cultured Ditaxisheterantha seeds either by mechanical extraction, solvent static extraction, solvent dynamic extraction, or alternatively by applying the following combined processes, such as solvent static and mechanical extraction, and solvent mechanical and dynamic extraction. The oleoresin may be used as a natural dye in the preparation of food, cosmetics, or textiles (Lopez and Cervantes Citation2007). Refined apricot kernel oil, comprising of lycopene, β-carotene and zeaxanthin can be used for health care products (B. Yuan and Fan Citation2016). Virgin oleoresins with four time higher concentration of carotenoids than those obtained by conventional methods can be used in animal feed, food and nutraceuticals (Gómez et al. Citation2016). Maize bran oil rich in zeaxanthine (300–3000 ppm) was obtained using combination of tera-hertz radiation and sub-critical extraction (Qin et al. Citation2014).
Marigold flowers and plants are used for obtaining lutein, zeaxanthin and rare carotenoids. (All-E)-lutein, (All-E)-zeaxanthin, lutein in Z configuration, β-carotene and cryptoxanthin from marigold flower petals have been proposed for use in food products as supplements (Swaminathan and Madavalappil Citation2008). A patent reports a method for separating and purifying all- Z high-purity lutein esters giving a yield exceeding 80% (X. Zhang et al. Citation2012). The separation and purification of fatty acid esters of lutein from marigold oil resin with total yield of 68.4% was reported in another patent (Xinde et al. Citation2006). The content of xanthophyll fatty acid ester was 70%–80%, and the content of all-E xanthophyll ester was 90%–95%. A method for separating and purifying all-E xanthophyll powder from marigold oleoresin (content >92%) resulting in total carotenoid content of >98% is described in patent by Yueqi et al. (Citation2010). Raw pot marigold flowers were thermo-oxidized in the presence of oxygen at 80–90 °C for 1.5–2.0 h subsequently ground and carotenoids were extracted with warmed ethyl alcohol for 1.5–2.0 h (Vashkevych, Stepnevcka, and Laptieva Citation2010). Another patent reports a method where zeaxanthin crystals were obtained from the berries of Lycium Chinese Mill, known as Chinese boxthorn (Khachik Citation2005).
There are a number of patents which describe the extraction of carotenoids from various seeds and leaves. Carotenoids from the Suaeda salsa seeds were extracted using solvent extraction yielding 194.47 mg/kg DW carotenoids (Guo, Suo, and Wang Citation2017). Seabuckthorn (Hippophae rhamnoides) seeds extract contains up to 92.5 mg/kg carotenoids (Zhao Citation2013). Fresh golden sweet willow (Melaleuca bracteata) leaves also give high extraction yield of carotenoids (Lin and Wang Citation2014). Carotenoids could be extracted from waste oil sludge of a ginkgo leaf (Shi Citation2015). An extraction method for obtaining lutein from green tea is patented in KR20140082601 (Choung et al. Citation2014). Supercritical CO2 extraction and ethanol solvent extraction was used for extracting carotenoids and flavonoids from purple operilla leaves which could be used in drinks (Liu et al. Citation2005).
Different plants can be used for carotenoid extraction such as Hamamelis virginiana L. which was used for the preparation of the fading-prevention agent, produced by extracting carotenoids with water or a hydrated alcohol, and can be used in foods and drink (Kondo, Tamura, and Miyagawa Citation1994). A method was reported for extracting carotenoids from Chinese wolfberry by microwaves with ethanol, where the stability of pigment is well maintained, and the extraction rate of 87.4% is favorable for application in the food industry (Xiuying et al. Citation2010). Wild chrysanthemum (Chrysanthemum indicum L.) carotenoids were extracted with ultrasonic assisted method and the obtained extract is suitable for food industry applications (Qiaosheng, Haijin, and Li Citation2011). Physalis persistent calyx (red girl, hanging lamp, lantern grass) carotenoids were extracted using supercritical CO2 yielding 15.96 mg/g of carotenoids, comprising of zeaxanthin (75%), followed by β-cryptoxanthin (16%) and lutein (8%) (Zhen, Qing, and Meijing Citation2011).
There are examples of patents where sludge and damp biomass were used for isolation of carotenoids. Water extract and the ethanol extract of the persimmon sludge fermented by microorganisms were reported to have carotenoid contents of 0.18 μg/mL and 6.79 μg/mL, respectively (Han and Jeong Citation2016). A method was patented for extracting carotenoids from damp biomasses which comprises milling and biomass drying, mixing extracting agents with oil or fat, thermal treatment followed by extraction of carotenoids under low pressure (Ozegowski et al. Citation2006).
There are patents for carotenoid extraction using organic solvents, supercritical CO2 extraction, and different assisted extraction methods. The food industry has long been unable to efficiently utilize carotenoids, primarily for their color, due to stability and solubility issues during their isolation. A number of patents that offer a solution through carotenoid synthesis and synthetic production have been a cost-effective alternative. However, consumer demands for natural additives has resulted in industry developing methods for the extraction of natural dyes, including carotenoids, and their application in food systems.
Carotenoids from microalgae were isolated by supercritical CO2 (Reyes, Nuñez, and Del Valle Citation2015), while Spirulina biomass was extracted with the mixture of solvents (Georgiovic et al. Citation2014). Different algae were used for the extraction of particular carotenoids, like in the patents listed below. There is a patent reporting the simultaneous extraction of chlorophyll and carotenoids from Spirulina (Wei and Ma Citation2014). Thermophilic strain of Chlorella pyrenoidos is used for production of lutein (Roche Citation1965), or chlorella algae powder for the extraction of xanthophylls (Hai et al. Citation2008). Fixation of astaxanthin, extracted from photosynthetic microalgae has also been patented (Lee and Park Citation2010).
Additionally, besides plants, a wide range of microorganisms for production of carotenoids have been eproposed as a natural source of these compounds. For example, patent by Treganowan et al. (Citation2017) presents the extraction of carotenoids from microorganisms with non-polar solvent and additional washing with aqueous solution of Brønsted acid (phosphoric acid, and citric acid, tartaric acid and maleic acid). The extracted carotenoid was found to have higher purity in the crystal form, which is due to removal of undesired lipophilic residues during the washing step. Numerous patents report processes for isolating carotenoids from selected strains of yeasts, fungi and bacteria, using solvent or supercritical fluid extraction. The resulting extracts are either pure, crystalline single carotenoid or a high yield mixture of carotenoids. They can be used for food and feed. Thus, astaxanthin has been isolated from Phaffia rhodozyma (X. Du et al. Citation2014; Kagan and Braun Citation2003), while some mutants of the same strain were used for isolation of β-carotene (Girard, Javelot, and Vladescu Citation2001). Astaxanthin is also isolated from the yeast of the genus Xanthophyllomyces (Du et al. Citation2014; Kanetani and Kinoshita Citation2016). High purity β-carotene and lycopene crystals are obtained from fungal biomass Blakeslea Sp. (Joseph and Anandane Citation2013; Wu et al. Citation2013). β-Carotene could be obtained from Rhodotorula mucilaginosa (Arken, Yang, and Yi Citation2007) and by fermenting selected bacterial strains (Van Keulen et al. Citation2010). Lycopene is obtained also from mucoral fungi such as Blakeslea, Choanephora, or Phycomyces (Estrella De Castro et al. Citation2001). Deinoxanthin is a carotenoid that can be obtained from the microorganism Deinococcus radiodurans (Sazykin, Sazykina, and Chistjakov Citation2013). Waste can also be used for producing natural carotenoids, like in the patent where soybean whey was used for cultivation of Deinococcus radiodurans (Yuejin et al. Citation2012), or trimmings of canned yellow peaches for yeast, Rhodotorula benthica (Shao Citation2016). In relation to this a culture method of high-yield carotenoid from Cordyceps militaris (Zhou and Ding Citation2014) has also been patented. Processes for isolation of a carotenoid from a carotenoid-producing microorganism with high content of highly pure, low-cost and safe carotenoid using supercritical CO2 or edible solvents extraction are reported in a number of patents (Bridges et al. Citation2003; Kanaya, Kinoshita, and Hirano Citation2014; Sibeijn, Wolf, and Schaap Citation2003; Treganowan et al. Citation2017). To increase the yield of carotenoid, the addition of glycerol in the fermentation medium is patented (J. Wang Citation2007).
Considering the low polarity of carotenoids, non-polar organic solvents have to be used for their effective isolation. New environmentally friendly solvents are being increasingly studied. Ishida et al. (Citation2009) patented a method for extraction of carotenoids from dry plant material using ethyl lactate. After removal of the dissolved carotenoids, the extraction solvent can be recycled for further use. A solvent-free process has been patented without any precipitation aid for preparing a plant extract in the form of a lipid-protein complex enriched in carotenoids and other antioxidants from tomato, carrot, apricot, guava, watermelon, papaya, pink grapefruit, mango, melon, cabbage, broccoli, lettuce, parsley, spinach, watercress or pepper. A crushed carotenoid-containing material is incubated the juice is recovered after the separation of plant solids, and then subjected to a heat treatment, while the precipitate is then recovered from the heated liquid phase after the second separation, to obtain a colored sludge extract which can be obtained in powdery form. The proposed process is simple, cost efficient, safe and it gives higher yield of extracted active compounds in comparison with other solvent-free methods. Based on dry matter, the plant extract can contain 1%–10% of carotenoids and 0.05%–0.5% of other antioxidants. These extracts could be used for the preparation of food or pet food products (Daury and Juillerat Citation2000). CO2 in a supercritical or subcritical state is used for extraction of carotenoids from plant tissue, including a technique for producing a carotenoid dye and high quality β-cryptoxanthin (Inomata et al. Citation2007). Carotenoids from red pepper (Capsicium annuumL.) were extracted using supercritical fluid extraction, yielding 50 to 67 g/kg of carotenoids, at optimum conditions (Choi et al. Citation2013). Extraction of carotenoid pigments from yellow pepper with aqueous alcohol is described in patent UA69607 (Vashkevych, Stepnevska, and Yudych Citation2012). Carotenoids from paprika were simultaneously extracted and concentrated in a series of mixing and high temperature and pressure mechanical pressing steps using edible solvent and a counter-current extraction procedure (Todd Citation2000).
Enzymes could be used in the extraction of carotenoids to increase the efficiency or to de-esterify the carotenoids. Cell wall degrading enzymes are used to increase extraction efficiency of carotenoids from Capsicum. An esterase, effective in de-esterifying the fatty acid from esterified carotenoids in red pepper, thereby increasing the fraction of free carotenoids in the oleoresin, have also been reported in the patent (Kanner, Granit, and Levy Citation2005). Enzymes including cellulase are used in a patent for obtaining the lycopene concentration of 73.3 ppm from tomato peel (Ferrari et al. Citation2013).
Carotenoid hollow fiber membrane liquid-phase micro-extraction method can be used for isolation of various carotenoids. They can enter the receptor solution in the hollow fiber cavity using vortex mixing, ultrasonic treatment, auxiliary stirring of magnetic fluids. The proposed method can shorten the extraction time and it is simple, effective and eliminates interfering substances (Meng et al. Citation2014).
Patents related to carotenoid formulations
There are many forms of carotenoid containing formulations available, including solid, liquid, paste or gel formulations. Solid formulations have dusty appearance or have a tendency for caking, while liquid formulations could be in homogenous and precipitate. When the matrix for the carotenoid formulation is wax and/or fat these disadvantages can be avoided. To produce wax and/or fat based carotenoid beadlets a spray chilled process, which is a mild procedure, is described in patent No. WO2014060566 (Badolato Boenisch, and Schlegel Citation2014). Matrix with different lipophilicity, such as starch and/or starch derivatives (Schaffner Citation2006; Scialpi Citation1987), wax(es) and/or fat(s) (Schlegel and Badolato Boenisch Citation2012), have been used as carrier for carotenoids in other patents. Color saturation as well as the color stability in these beadlets is reported to be very good.
Carotenoid utilization in pharmaceutical products, as coloring agents in foodstuffs and as feed additives is limited by problems which include water-insolubility, high melting point as well as the size of the particles in the final formulation. These problems lead to poor bioavailability of carotenoids from the final product. Production of carotenoid particles in the nanometer range is essential to achieve a suitable bio-availability and color yield. Chinese patent 101549273B describes a method of preparing nano-dispersed high-all-E-carotenoid microcapsules (Zhirong et al. Citation2009). Crystal forms of (all-E)-carotenoids were ground to the size 2–5 μm, then suspended in dichloromethane and ethanol or isopropanol, crystalized again in a supergravity rotating packed bed crystallization device, concentrated and mixed with aqueous solution containing antioxidant and protective colloid, and then spray-dried. The authors claim that, due to the nano-sized particles and high content of trans isomers (>90%), bioavailability of carotenoids in this formulation is high.
Carotenoid instability, i.e., sensitivity to heat treatments, oxygen and other oxidizing agents, is one of the key issues in their formulation and application. Encapsulation has been proposed as a technique of choice that protects sensitive phytochemicals, extends their shelf life and accompanied biochemical functionalities, and eases their incorporation into certain food products due to prevention of lumping, improving flow ability, compression and mixing properties, reducing core particle dustiness and modifying particle density (Tumbas Šaponjac et al. Citation2017). Gelatin was used as a film forming component and protective colloid, disabling oxygen permeability. In this patent, carotenoids are dispersed in edible oil while the water content in the microcapsules was up to 10% providing multi-core structure and high stability toward carotenoid leaking and capsule breakdown. Carrier (coating) agents for the carotenoid capsule formulations are mostly gelatin (Akamatsu et al. Citation1998; Antoshkiw, Cannalonga, and Koff Citation1976; Chiavazza, Dollat, and Fayard Citation2004), starch/modified starch (Estrella De Castro et al. Citation2004; Gellenbeck Citation1998; Leuenberger, Schlegel, and Voelker Citation2004; Musaeus and Jensen Citation2007), gum arabic (Klingenberg Citation2005), pectin (Carle, Schieber, and Mutter Citation2006), soy protein (Runge, Lueddecke, and Pfeiffer Citation2002). In a Japanese patent 5368316B2, liquid carotenoid formulation was based on a protective colloid (casein, caseinate, bovine, pig or fish gelatin, modified starch and cellulose) and water miscible alcohol (Unknown authors Citation2013). This formulation can be added directly to aqueous or non-aqueous preparations.
For the formulation of fat-soluble carotenoid-containing functional health ingredients, addition of carotenoid extract to a protein (e.g., bovine, swine or fish gelatin or hydrolyzed gelatin), cross-linked with a reducing substance (fructose, glucose, lactose, maltose, xylose, arabinose, ribose, invert sugar or high fructose or glucose sirups, glutaraldehyde) and a solid vegetable fat (hydrogenated sunflower, rapeseed, castor bean, cotton seed, coconut and palm oils) was proposed in Patent EP1418822 (Leuenberger Citation2004). The mixture is homogenized by conventional techniques (agitating, high-pressure homogenization, high-shear emulsification, etc.) and the emulsion converted into a powder such as granules or beadlets, by spraying onto a bed of starch/modified starch. The cross-linking may be accomplished by heat-treatment (60–100 °C for 10–60 min) or with enzymes (e.g., transglutaminase).
Patents related to carotenoids in food formulation
Carotenoids extracted from different sources have been added to human food in various formulations, primarily due to their attractive color, and more recently, due to their potential health benefits. Many innovative formulations have been protected by patent applications.
Chinese wolfberry (Lycium barbarum) was used for the extraction and formulation of a fermented beverage with increased content of carotenoids. As described in patent (Ma et al. Citation2017), the content of Chinese wolfberry for the preparation of the fermented drink is 5%, wt/wt, and the content of carotenoids in Chinese wolfberry extract is 58.44 mg/L. Additionally, a method for obtaining carotenoid monomer pigment, mainly zeaxanthin, from wolfberries with high extraction rate and high purity and with low content of solvent residues was patented (Liang and Shao Citation2017).
Rhodoxanthin and optionally β-carotene or β-apo-8-carotenal can be used for preparing an edible coating in confectionary, such as chocolate lentils. This edible coating has preferably a red value a* of at least 36 at the CIELAB color scale, with rhodoxanthin in the concentration ranging from 20 to 60 ppm, and the average particle size from 250 to 320 nm. In the formulation the rhodoxanthin is embedded in a matrix of a protective hydrocolloid consisting of modified food starches. Additionally, water- and/or fat-soluble antioxidants and edible oils may be present in the formulation. (Grass and Hitzfeld Citation2016).
Lycopene-enriched meat and fish products, derived from the addition of a dry and ground tomato peel, which originated as a by-product of tomato processing and from surplus of fresh tomato production, have been patented (Calvo Rodriguez et al. Citation2008).
Papaya, a rich source of β-carotene, vitamin C and other nutrients, was used in a multi-nutrient mix, in the manufacture of biscuits for children. This invention relates to the development of a process technology without any artificially added fortifications. Pre-processing step for papaya included deseeding and peeling. The pulp is manually extracted and dried in trays at 50–70 °C for 12–18 h. All ingredients, including papaya, are then ground to a particle size less than 200 µm. It is reported that these biscuits are nutritious, have a good mouth feel, color, texture and other organoleptic qualities (Agrahar Murugkar Citation2015).
Another patent describes colloidal dispersed β-carotene added in the amount of 8.7 × 10−5–6.02 × 10−3 % (wt/wt) to formulate a functional “soy milk” drink as a prophylactic and gerodietetic product (Kochetkova et al. Citation2004).
Zeaxanthin formulations, containing the 3 R-3'R stereoisomer of zeaxanthin can be produced in large quantities and at a low cost, to obtain viscous oily fluid containing 5%–20% zeaxanthin, by means of a simple solvent extraction process. This isomer of zeaxanthin can help treat and prevent macular degeneration, one of the leading causes of blindness and vision loss, especially among the elderly. Formulation is patented for application in soups, salads, drinks, or other foods, and also as the ingestible tablets (Garnett, Guerra-Santos, and Gierhart Citation2003).
The maximum content of natural carotenoids in fortified edible vegetable oil can reach the level of 1.6–6.1 g/L when new techniques of ultrasonic and microwave treatments were used (Y. Li Citation2016).
The process for concentration and purification of extracts obtained from cashew pseudo-fruit wastes and making products with high content of carotenoid components (auroxanthin, mutatoxanthin, lutein, zeaxanthin, antheraxanthin, β-cryptoxanthin, (13Z)-β-carotene, α-carotene and β-carotene) was patented. Concentrate has a potential use as a coloring agent in food and feed; it is applicable in the areas of ready-to-drink juices and beverages due to its significant solubility in water (Pinto De Abreu et al. Citation2019).
The production of an ingredient rich in fucoxanthin and a fucoxanthin derivative obtained from seaweed having an effect of inhibiting neutral fat absorption is described in patent (Matsumoto et al. Citation2008) which includes high polarity carotenoids as active ingredients, with the use in foods, drinks, supplements, pet foods, sanitary materials, cosmetics or chemicals.
Patents relating to carotenoids in feed formulation
As already commented, carotenoids are used in feeds to improve the color of animal-derived foods or for improving animal nutrition. Nettle was added in the duck cramming food, either fresh, freeze-dried, or as an extract or juice. Animals were fed the equivalent of 40 to 60 g of crushed fresh nettle for at least part of the feeding period, in order to increase the content of β-carotene and silicon which results in improved properties of the liver including the rendering and melting rate, the organoleptic properties, the content of vitamin A, vitamin E, β-carotene and silicon. The invention has applications in the production of “foiegras” and stuffed poultry meat, in particular ducks but also geese (Candau Citation2008).
Xanthophyll from chicory plant was one out of four components added into mixed feed for Chinese turtle as a quality improving agent. A mixture of four kinds of active components is added in the amount of 0.01%–1% (wt/wt) (A. Chen, Yang, and Hong Citation2005).
A fat-soluble carotenoid complex produced from echinoderms, crustacea and other hydro bios and waste from reprocessing has been patented. Raw materials are ground to particle size of 0.1–50 mm, extracted with heated fish or vegetable oil in ratio of 1:1–1:3, under continuous intense agitation, at 15–40 °C, for 30–40 min. The carotenoid complex fat extract is then separated. This carotenoid enriched fat extract is useful as a feed supplement for salmon, possessing health and prophylaxis properties (Muhin et al. Citation2005).
Conclusions
Carotenoids are widely distributed dietary components, some of which can be converted in vitamin A, an essential nutrient for humans. Apart from this key distinctive characteristic they are more versatile than other so-called food bioactives, as they are also natural pigments, antioxidants and can provide health and cosmetic benefits, hence their growing importance in the context of functional foods, nutraceuticals and nutricosmetics. They are widely distributed and their contents have been thoroughly studied in common foods, as well as in exotic, underutilized or even non-domesticated plants. The studies of such uncommon sources has resulted over the last decades in the identification of sources with very high levels of health-promoting carotenoids and should continue to be a key research line within the field in accordance with international recommendations and efforts to make a better use of biodiversity in the context of sustainability and food security. Indeed, any new research on carotenoids in the context of agro-food should be completely aligned with the need of ensuring food security in a sustainable manner while contributing to the reduction of diseases related to nutrition. Key to this global objective are the United Nations Sustainable Development Goals (SDGs) (https://www.un.org/sustainabledevelopment/sustainable-development-goals/) and the new model of circular economy, in which resources are re-used efficiently to contribute to sustainability, which is already a priority in the political agenda.
Therefore research on sustainable agronomic, postharvest, technological and any other aproches to produce quality carotenoid-containing foods (including the exploitation of by-products, waste or any industry effluent) should continue being encouraged. Studies on the impact of climate change on the food levels of carotenoids appear especially timely. This review shows that there is a large body of studies dealing with these aspects and that non-thermal and other environmentally friendly approaches are gaining importance. In this regard, ethyl lactate, a biodegradable environmentally friendly solvent, produced from the fermentation of carbohydrate feedstock can offer advantages for the extraction of carotenoids.
In relation to the impact of technological or even culinary practices in carotenoids, aspects other than the mere content should be addressed, as evidence is accumulating that, in some cases, the decrease of the levels of carotenoids can be accompanied by an increase in their bioavailability due to structural changes in the matrix, facilitating their release during digestion. More holistic approaches assessing how these practices affect aspects of food quality (for instance safety, sensory or nutritional quality) related to carotenoids should be therefore encouraged in the future. At this point it is important to note that apparent increases in the net carotenoid content of foods after these have been subjected to different technological treatments continue to be reported. Such data must be interpreted with caution and extreme care must be taken before conducting such studies to ensure that the extraction methods used during their analysis are appropriate. The influence of weight loss or gain during such treatments (for instance due to water loss or gain) must also be adequately considered and appropriate formula to calculate carotenoid retention must be used.
Apart from foods, the search and exploitation of other sources, above all in aquatic environments can lead to important innovations. Despite some microalgae are being exploited for the commercial production of carotenoids, their abundance and wide variety will continue to offer many opportunities of research and innovation. Similarly, aquatic animals that are known to contain carotenoids with unusual structures (for instance sponges) have been scarcely studied in the context of the production of carotenoid-containing products.
In relation to the analysis of carotenoids, important advances have been made in the last years, including new techniques of extraction and analysis that can offer advantages including higher throughput, less consumption of solvents, improved separation and detection, etc. Moreover, chemometric analysis of analytical data is currently used for discrimination of cultivars or to reveal the changes of carotenoid profiles during ripening. Being unusual carotenoids due to their lack of color, phytoene and phytofluene have been largely ignored in most studies in the context of food science and technology, although it is now clear that they are major dietary carotenoids that are present in human fluids and plasma at levels comparable or superior to the carotenoids typically studied in relation to health. Furthermore evidence is accumulating that they can provide health and even cosmetic benefits. The analytical methods applied to carotenoid analysis in foods should always therefore take into account these compounds. On the other hand, apocarotenoids (formed enzymatically or not from the major dietary carotenoids) are attracting increased attention as they may be involved in biological actions. They have been shown to occur at considerable lower levels, such that new developments in carotenoid analytical methods should ideally try to target the identification and quantification of these compounds, which are usually very unstable.
As discussed, reliable data on carotenoid contents and intakes are essential for different purposes, some of which converge into the establishment of recommended carotenoid intakes for the promotion of health. Although important advances have been made in recent years, there is much room for improvement. Thus, aspects including the consensus of carotenoids to be included or the harmonization of protocols for the collection of data need should be carefully considered in future studies. As far as the overview of carotenoid patents carried out, it is clear that these compounds continue offering many opportunities for innovation. Indeed, of the over 700 carotenoids that have been fully and appropriately characterized, 10–20 (mostly, major dietary carotenoids and within them those that are typically found in humans) are being extensively studied. Chances that there are many carotenoids that can be used as colorants in foods or feeds or to promote animal welfare are many. Undeniably, there is still plenty of room to advance research and innovation with carotenoids, this being the leitmotif of the COST Action EUROCAROTEN (www.eurocaroten.eu - https://www.cost.eu/actions/CA15136/#tabs|Name:overview).
Acknowledgments
This article is based upon work from COST Action (European network to advance carotenoid research and applications in agro-food and health, EUROCAROTEN, CA15136, www.eurocaroten.eu, https://www.cost.eu/actions/CA15136/#tabs|Name:overview) supported by COST (European Cooperation in Science and Technology, http://www.cost.eu/).
Disclosure statement
A. J. Meléndez-Martínez was a member of the advisory board of IBR—Israeli Biotechnology Research, Ltd. There are not any financial or other relationships that might create a conflict of interest for the authors apart from the one declared.
References
- Aas, G. H., B. Bjerkeng, B. Hatlen, and T. Storebakken. 1997. Idoxanthin, a major carotenoid in the flesh of Arctic charr (Salvelinus alpinus) fed diets containing astaxanthin. Aquaculture 150 (1–2):135–42. doi: 10.1016/S0044-8486(96)01458-5.
- Adeyemi, K. D., A. B. Sabow, M. Ebrahimi, A. A. Samsudin, and A. Q. Sazili. 2016. Fatty acid composition, cholesterol and antioxidant status of infraspinatus muscle, liver and kidney of goats fed blend of palm oil and canola oil. Italian Journal of Animal Science 15 (2):181–90. doi: 10.1080/1828051X.2016.1158081.
- Agabriel, C., A. Cornu, C. Journal, C. Sibra, P. Grolier, and B. Martin. 2007. Tanker milk variability according to farm feeding practices: Vitamins A and E, carotenoids, color, and terpenoids. Journal of Dairy Science 90 (10):4884–96. doi: 10.3168/jds.2007-0171.
- Agócs, A., V. Nagy, Z. Szabó, L. Márk, R. Ohmacht, and J. Deli. 2007. Comparative study on the carotenoid composition of the peel and the pulp of different citrus species. Innovative Food Science & Emerging Technologies 8 (3):390–4.
- Agrahar Murugkar, D. 2015. Process technology for multi-nutrient composite mix for biscuits. Patent No. IN2435, filed July 22, 2013, and issued June 19, 2015.
- Ahmad, M., and A. R. Cashmore. 1993. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366 (6451):162–6. doi: 10.1038/366162a0.
- Ahmad, F. T., D. E. Mather, H. Y. Law, M. Li, S. A. J. Yousif, K. J. Chalmers, R. E. Asenstorfer, and D. J. Mares. 2015. Genetic control of lutein esterification in wheat (Triticum aestivum L.) grain. Journal of Cereal Science 64:109–15. doi: 10.1016/j.jcs.2015.05.007.
- Akamatsu, T., R. Yasue, K. Kiyama, and N. Hara. 1998. Microcapsules of the multi-core structure containing natural carotenoid. Patent No. US5780056, filed September 17, 1996, and issued July 14, 1998.
- Al-Delaimy, W. K., N. Slimani, P. Ferrari, T. Key, E. Spencer, I. Johansson, G. Johansson, I. Mattisson, E. Wirfalt, S. Sieri, et al. 2005. Plasma carotenoids as biomarkers of intake of fruits and vegetables: Ecological-level correlations in the European Prospective Investigation into Cancer and Nutrition (EPIC). European Journal of Clinical Nutrition 59 (12):1397–408. doi: 10.1038/sj.ejcn.1602253.
- Almeida, A., C. Serra, and M. G. Dias. 2017. Efeito da sazonalidade no teor de carotenoides em frutos e produtos hortícolas consumidos em Portugal. Boletim Epidemiológico Observações 6 (20):33–6.
- Alvarez, J. B., L. M. Martin, and A. Martin. 1999. Genetic variation for carotenoid pigment content in the amphiploid Hordeum chilense × Triticum turgidum conv. durum. Plant Breeding 118 (2):187–9. doi: 10.1046/j.1439-0523.1999.118002187.x.
- Álvarez, R., A. J. Meléndez-Martínez, I. M. Vicario, and M. J. Alcalde. 2014. Effect of pasture and concentrate diets on concentrations of carotenoids, vitamin A and vitamin E in plasma and adipose tissue of lambs. Journal of Food Composition and Analysis 36 (1–2):59–65. doi: 10.1016/j.jfca.2014.08.001.
- Álvarez, R., A. J. Meléndez-Martínez, I. M. Vicario, and M. J. Alcalde. 2015. Carotenoids and fat-soluble vitamins in horse tissues: A comparison with cattle. Animal 9 (7):1230–8. doi: 10.1017/S1751731115000415.
- Al-Yafeai, A., A. Malarski, and V. Böhm. 2018. Characterization of carotenoids and vitamin E in R. rugosa and R. canina: Comparative analysis. Food Chemistry 242:435–42. doi: 10.1016/j.foodchem.2017.09.070.
- Ambati, R. R., S. M. Phang, S. Ravi, and R. G. Aswathanarayana. 2014. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications-a review. Marine Drugs 12 (1):128–52. doi: 10.3390/md12010128.
- Amoozgar, A., A. Mohammadi, and M. R. Sabzalian. 2017. Impact of light-emitting diode irradiation on photosynthesis, phytochemical composition and mineral element content of lettuce cv. Photosynthetica 55 (1):85–95. doi: 10.1007/s11099-016-0216-8.
- Amorim-Carrilho, K. T., A. Cepeda, C. Fente, and P. Regal. 2014. Review of methods for analysis of carotenoids. TrAC Trends in Analytical Chemistry 56:49–73. doi: 10.1016/j.trac.2013.12.011.
- Andersson, S. C., M. E. Olsson, E. Johansson, and K. Rumpunen. 2009. Carotenoids in sea buckthorn (Hippophae rhamnoides L.) berries during ripening and use of pheophytin a as a maturity marker. Journal of Agricultural and Food Chemistry 57 (1):250–8. doi: 10.1021/jf802599f.
- Anese, M., G. Mirolo, P. Beraldo, and G. Lippe. 2013. Effect of ultrasound treatments of tomato pulp on microstructure and lycopene in vitro bioaccessibility. Food Chemistry 136 (2):458–63. doi: 10.1016/j.foodchem.2012.08.013.
- Antoshkiw, T. W., M. A. Cannalonga, and A. Koff. 1976. Water dispersible carotenoid preparations and processes thereof. Patent No. US3998753, filed August 13, 1974, and issued December 21, 1976.
- Arathi, B. P., P. R. R. Sowmya, K. Vijay, V. Baskaran, and R. Lakshminarayana. 2015. Metabolomics of carotenoids: The challenges and prospects–A review. Trends in Food Science & Technology 45 (1):105–17.
- Arken, R., Y. Yang, and X. Yi. 2007. Rhodotorula mucilaginosa for producing beta-caroten, beta-caroten and its production method. Patent No. CN101008000, filed January 25, 2006, and issued August 1, 2007.
- Arnold, L. E., N. Lofthouse, and E. Hurt. 2012. Artificial food colors and attention-deficit/hyperactivity symptoms: Conclusions to dye for. Neurotherapeutics 9 (3):599–609. doi: 10.1007/s13311-012-0133-x.
- Ashokkumar, K., M. Diapari, A. B. Jha, B. Tar’an, G. Arganosa, and T. D. Warkentin. 2015. Genetic diversity of nutritionally important carotenoids in 94 pea and 121 chickpea accessions. Journal of Food Composition and Analysis 43:49–60. doi: 10.1016/j.jfca.2015.04.014.
- Atienza, S. G., J. Ballesteros, A. Martín, and D. Hornero-Méndez. 2007. Genetic variability of carotenoid concentration and degree of esterification among tritordeum (xTritordeum Ascherson et Graebner) and durum wheat accessions. Journal of Agricultural and Food Chemistry 55 (10):4244–51. doi: 10.1021/jf070342p.
- Babu, C. M., R. Chakrabarti, and K. R. S. Sambasivarao. 2008. Enzymatic isolation of carotenoid-protein complex from shrimp head waste and its use as a source of carotenoids. LWT - Food Science and Technology 41 (2):227–35. doi: 10.1016/j.lwt.2007.03.006.
- Badolato Boenisch, G., and B. Schlegel. 2014. Beadlets comprising carotenoids. Patent No. WO2014060566, filed October 18, 2013, and issued April 24, 2014.
- Baiano, A. 2020. Edible insects : An overview on nutritional characteristics, safety, farming, production technologies, regulatory framework, and socio-economic and ethical implications. Trends in Food Science & Technology 100:35–50.
- Ballet, N., J. C. Robert, and P. E. V. Williams. 2000. Vitamins in forages. In Forage evaluation in ruminant nutrition, eds. D. I. Givens, E. Owen, H. M. Omed, and R. F. E. Axford, 399–431. Wallingford, UK: CABI.
- Bantis, F., K. Karamanoli, A. Ainalidou, K. Radoglou, and H. I. A. Constantinidou. 2018. Light emitting diodes (LEDs) affect morphological, physiological and phytochemical characteristics of pomegranate seedlings. Scientia Horticulturae 234:267–74. doi: 10.1016/j.scienta.2018.02.065.
- Bantis, F., S. Smirnakou, T. Ouzounis, A. Koukounaras, N. Ntagkas, and K. Radoglou. 2018. Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs). Scientia Horticulturae 235:437–51. doi: 10.1016/j.scienta.2018.02.058.
- Basim, D. G. B., and A. Nice. 2017. Process and method for optimal recovery of carotenoids from plants. Patent No. WO2017111761, filed December 26, 2016, and issued June 29, 2017.
- Behsnilian, D., and E. Mayer-Miebach. 2017. Impact of blanching, freezing and frozen storage on the carotenoid profile of carrot slices (Daucus carota L. cv. Nutri Red). Food Control. 73:761–7. doi: 10.1016/j.foodcont.2016.09.045.
- Beltrán, B., R. Estevez, C. Cuadrado, S. Jimenez, and B. A. Olmedilla. 2012. Carotenoid data base to assess dietary intake of carotenes, xanthophyls and vitamin A; its use in a comparative study of vitamin A nutritional status in young adults. Nutricion Hospitalaria 27 (4):1334–43. doi: 10.3305/nh.2012.27.4.5886.
- Beltrán-de-Miguel, B., R. Estévez-Santiago, and B. Olmedilla-Alonso. 2015. Assessment of dietary vitamin A intake (retinol, α-carotene, β-carotene, β-cryptoxanthin) and its sources in the National Survey of Dietary Intake in Spain (2009–2010). International Journal of Food Sciences and Nutrition 66 (6):706–12. doi: 10.3109/09637486.2015.1077787.
- Benmeziane, A., L. Boulekbache-Makhlouf, P. Mapelli-Brahm, N. Khaled Khodja, H. Remini, K. Madani, and A. J. Meléndez-Martínez. 2018. Extraction of carotenoids from cantaloupe waste and determination of its mineral composition. Food Research International (Ottawa, Ont.) 111:391–8. doi: 10.1016/j.foodres.2018.05.044.
- Bergquist, S. Å., U. E. Gertsson, and M. E. Olsson. 2006. Influence of growth stage and postharvest storage on ascorbic acid and carotenoid content and visual quality of baby spinach (Spinacia oleracea L.). Journal of the Science of Food and Agriculture 86 (3):346–55. doi: 10.1002/jsfa.2373.
- Biehler, E., A. A. Alkerwi, L. Hoffmann, E. Krause, M. Guillaume, M. L. Lair, and T. Bohn. 2012. Contribution of violaxanthin, neoxanthin, phytoene and phytofluene to total carotenoid intake: Assessment in Luxembourg. Journal of Food Composition and Analysis 25 (1):56–65. doi: 10.1016/j.jfca.2011.07.005.
- Bijttebier, S. K., E. D'Hondt, N. Hermans, S. Apers, and S. Voorspoels. 2013. Unravelling ionization and fragmentation pathways of carotenoids using orbitrap technology: A first step towards identification of unknowns. Journal of Mass Spectrometry: JMS 48 (6):740–54. doi: 10.1002/jms.3203.
- Bijttebier, S. K., E. D'Hondt, B. Noten, N. Hermans, S. Apers, and S. Voorspoels. 2014. Ultra high performance liquid chromatography versus high performance liquid chromatography: Stationary phase selectivity for generic carotenoid screening. Journal of Chromatography. A 1332:46–56. doi: 10.1016/j.chroma.2014.01.042.
- Bjerkeng, B. 2000. Carotenoid pigmentation of salmonid fishes – Recent progress. In L. E. Cruz-Suárez, D. Ricque-Marie, M. TapiaSalazar, M. A. Olvera-Novoa, y R. Civera-Cerecedo, eds. Avances en nutrición acuícola V. Memorias del V Simposium Internacional de Nutrición Acuícola, 19–22 Noviembre, Mérida, Yucatán.
- Blandino, M., M. Alfieri, D. Giordano, F. Vanara, and R. Redaelli. 2017. Distribution of bioactive compounds in maize fractions obtained in two different types of large scale milling processes. Journal of Cereal Science 77:251–8. doi: 10.1016/j.jcs.2017.08.006.
- Bohn, T., G. J. McDougall, A. Alegría, M. Alminger, E. Arrigoni, A.-M. Aura, C. Brito, A. Cilla, S. N. El, S. Karakaya, et al. 2015. Mind the gap-deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites-a position paper focusing on carotenoids and polyphenols. Molecular Nutrition & Food Research 59 (7):1307–23. doi: 10.1002/mnfr.201400745.
- Bonaccorsi, I., F. Cacciola, M. Utczas, V. Inferrera, D. Giuffrida, P. Donato, P. Dugo, and L. Mondello. 2016. Characterization of the pigment fraction in sweet bell peppers (Capsicum annuum L.) harvested at green and overripe yellow and red stages by offline multidimensional convergence chromatography/liquid chromatography-mass spectrometry. Journal of Separation Science 39 (17):3281–391. doi: 10.1002/jssc.201600220.
- Borghesi, E., M. L. González-Miret, M. L. Escudero-Gilete, F. Malorgio, F. J. Heredia, and A. J. Meléndez-Martínez. 2011. Effects of salinity stress on carotenoids, anthocyanins, and color of diverse tomato genotypes. Journal of Agricultural and Food Chemistry 59 (21):11676–82. doi: 10.1021/jf2021623.
- Boukroufa, M., C. Boutekedjiret, and F. Chemat. 2017. Development of a green procedure of citrus fruits waste processing to recover carotenoids. Resource-Efficient Technologies 3 (3):252–62. doi: 10.1016/j.reffit.2017.08.007.
- Bourget, C. M. 2008. An introduction to light-emitting diodes. HortScience 43 (7):1944–6. doi: 10.21273/HORTSCI.43.7.1944.
- Bouzari, A., D. Holstege, and D. M. Barrett. 2015. Vitamin retention in eight fruits and vegetables: A comparison of refrigerated and frozen storage. Journal of Agricultural and Food Chemistry 63 (3):957–62. doi: 10.1021/jf5058793.
- Bravo, S., J. García-Alonso, G. Martín-Pozuelo, V. Gómez, V. García-Valverde, I. Navarro-González, and M. J. Periago. 2013. Effects of postharvest UV-C treatment on carotenoids and phenolic compounds of vine-ripe tomatoes. International Journal of Food Science & Technology 48 (8):1744–9. doi: 10.1111/ijfs.12146.
- Bravo, S., J. García-Alonso, G. Martín-Pozuelo, V. Gómez, M. Santaella, I. Navarro-González, and M. Jesús Periago. 2012. The influence of post-harvest UV-C hormesis on lycopene, β-carotene, and phenolic content and antioxidant activity of breaker tomatoes. Food Research International 49 (1):296–302. doi: 10.1016/j.foodres.2012.07.018.
- Brazaityté, A., S. Sakalauskienė, G. Samuolienė, J. Jankauskienė, A. Viršilė, A. Novičkovas, R. Sirtautas, J. Miliauskienė, V. Vaštakaitė, L. Dabašinskas, et al. 2015. The effects of LED illumination spectra and intensity on carotenoid content in Brassicaceae microgreens. Food Chemistry 173:600–6. doi: 10.1016/j.foodchem.2014.10.077.
- Brazaityté, A., R. Ulinskaité, P. Duchovskis, G. Samuoliené, J. B. Siksnianienė, J. Jankauskiené, G. Sabqieviené, K. Baranouskis, G. Staniené, G. Tamulaitis, et al. 2006. Optimization of lighting spectrum for photosynthetic system and productivity of lettuce by using light-emitting diodes. Acta Horticulturae 711 (711):183–8. doi: 10.17660/ActaHortic.2006.711.22.
- Bridges, T. L., W. Nordhausen, M. Olaizola, and P. Walliander. 2003. Edible solvent extraction of carotenoids from microorganisms. Patent No. US2003054070, filed September 7, 2001, and issued March 20, 2003.
- Britton, G., and F. Khachik. 2009. Carotenoids in food. In Carotenoids, eds. G. Britton, S. Liaaen-Jensen, and H. Pfander, vol. 5. Basel, Boston, Berlin: Birkhauser Verlag.
- Britton, G., S. Liaaen-Jensen, and H. Pfander. 2004. Carotenoids handbook.Basel, Boston, Berlin: Birkhauser Verlag.
- Brown, C. R. 2008. Breeding for phytonutrient enhancement of potato. American Journal of Potato Research 85 (4):298–307. doi: 10.1007/s12230-008-9028-0.
- BS EN 16104:2012. Food data. Structure and interchange format. ISBN 978 0 580 70792 6, January 2013.
- Burgie, E. S., A. N. Bussell, J. M. Walker, K. Dubiel, and R. D. Vierstra. 2014. Crystal structure of the photosensing module from a red/far-red light-absorbing plant phytochrome. Proceedings of the National Academy of Sciences of the United States of America 111 (28):10179–84. doi: 10.1073/pnas.1403096111.
- Burke, J. D., J. Curran-Celentano, and A. J. Wenzel. 2005. Diet and serum carotenoid concentrations affect macular pigment optical density in adults 45 years and older. The Journal of Nutrition 135 (5):1208–14. doi: 10.1093/jn/135.5.1208.
- Cacciola, F., P. Donato, D. Giuffrida, G. Torre, P. Dugo, and L. Mondello. 2012. Ultra high pressure in the second dimension of a comprehensive two-dimensional liquid chromatographic system for carotenoid separation in red chili peppers. Journal of Chromatography. A 1255:244–51. doi: 10.1016/j.chroma.2012.06.076.
- Cacciola, F., D. Giuffrida, M. Utczas, D. Mangraviti, P. Dugo, D. Menchaca, E. Murillo, and L. Mondello. 2016. Application of comprehensive two-dimensional liquid chromatography for carotenoid analysis in red mamey (Pouteria sapote) fruit. Food Analytical Methods 9 (8):2335–41. doi: 10.1007/s12161-016-0416-7.
- Calderón, F., B. Chauveau-Duriot, B. Martin, B. Graulet, M. Doreau, and P. Nozière. 2007. Variations in carotenoids, vitamins A and E, and color in cow’s plasma and milk during late pregnancy and the first three months of lactation. Journal of Dairy Science 90 (5):2335–46. doi: 10.3168/jds.2006-630.
- Calderón, F., B. Chauveau-Duriot, P. Pradel, B. Martin, B. Graulet, M. Doreau, and P. Nozière. 2007. Variations in carotenoids, vitamins A and E, and color in cow’s plasma and milk following a shift from hay diet to diets containing increasing levels of carotenoids and vitamin E. Journal of Dairy Science 90 (12):5651–64. doi: 10.3168/jds.2007-0264.
- Calvo Rodriguez, M. M., M. J. Rodriguez Castillo, J. G. Santa-Maria Blanco, M. D. Selgas Cortecero, and M. L. Garcia Sanz. 2008. Meat and fish products enriched with lycopen by means of addition of tomato peel. Patent No. WO2008155439, filed June 18, 2008, and issued December 24, 2008.
- Candau, A. 2008. Method for improving the properties of a product from duck, and duck cramming food for improving the properties of the liver and/or meat thereof. Patent No. WO2008132391, filed March 21, 2008, and issued November 6, 2008.
- Cantos, E., C. Garcia-Viguera, S. De Pascual-Teresa, and F. A. Tomas-Barberan. 2000. Effect of postharvest ultraviolet irradiation on resveratrol and other phenolics of cv. Napoleon table grapes. Journal of Agricultural and Food Chemistry 48 (10):4606–12. doi: 10.1021/jf0002948.
- Capanoglu, E., J. Beekwilder, D. Boyacioglu, R. Hall, and R. De Vos. 2008. Changes in antioxidant and metabolite profiles during production of tomato paste. Journal of Agricultural and Food Chemistry 56 (3):964–73. doi: 10.1021/jf072990e.
- Cardinault, N., M. Doreau, C. Poncet, and P. Nozière. 2006. Digestion and absorption of carotenoids in sheep given fresh red clover. Animal Science 82 (1):49–55. doi: 10.1079/ASC200514.
- Carle, R., A. Schieber, and S. Mutter. 2006. Novel compositions comprising carotenoids. Patent No. US2006110517, filed July 21, 2005, and issued May 25, 2006.
- Carrol, Y. L., B. M. Corridan, and P. A. Morrissey. 1999. Carotenoids in young and elderly healthy humans: Dietary intakes, biochemical status and diet-plasma relationships. European Journal of Clinical Nutrition 53 (8):644–53. doi: 10.1038/sj.ejcn.1600827.
- Carvalho, L. M. J. D., L. D. A. S. M. Smiderle, J. L. V. D. Carvalho, F. D. S. N. Cardoso, and M. G. B. Koblitz. 2014. Assessment of carotenoids in pumpkins after different home cooking conditions. Food Science and Technology (Campinas) 34 (2):365–70. doi: 10.1590/fst.2014.0058.
- Casal, J. J., and M. J. Yanovsky. 2005. Regulation of gene expression by light. The International Journal of Developmental Biology 49 (5–6):501–11. doi: 10.1387/ijdb.051973jc.
- Chandi, G. K., and B. S. Gill. 2011. Production and characterization of microbial carotenoids as an alternative to synthetic colors: A review. International Journal of Food Properties 14 (3):503–13. doi: 10.1080/10942910903256956.
- Chauveau-Duriot, B., M. Doreau, P. Nozière, and B. Graulet. 2010. Simultaneous quantification of carotenoids, retinol, and tocopherols in forages, bovine plasma, and milk: Validation of a novel UPLC method. Analytical and Bioanalytical Chemistry 397 (2):777–90. doi: 10.1007/s00216-010-3594-y.
- Chauveau-Duriot, B., D. Thomas, J. Portelli, and M. Doreau. 2005. Carotenoids content in forages: Variation during conservation. Renc Rech Ruminants 12:117.
- Chen, A., C. Yang, and Q. Hong. 2005. Quality improving agent for Chinese turtle and its use. Patent No. CN1647681, filed February 5, 2005, and issued August 3, 2005.
- Chen, B. H., and Y. C. Tang. 1998. Processing and stability of carotenoid powder from carrot pulp waste. Journal of Agricultural and Food Chemistry 46 (6):2312–8. doi: 10.1021/jf9800817.
- Chen, M., J. Chory, and C. Fankhauser. 2004. Light signal transduction in higher plants. Annual Review of Genetics 38:87–117. doi: 10.1146/annurev.genet.38.072902.092259.
- Chiavazza, V., J.-M. Dollat, and S. Fayard. 2004. Solid granules containing carotenoids. Patent No. EP1433387, filed December 26, 2002, and issued June 30, 2004.
- Chisté, R. C., and A. Z. Mercadante. 2012. Identification and quantification, by HPLC-DAD-MS/MS, of carotenoids and phenolic compounds from the Amazonian fruit Caryocar villosum. Journal of Agricultural and Food Chemistry 60 (23):5884–92. doi: 10.1021/jf301904f.
- Choi, H. D., Y. K. Park, I. W. Choi, Y. S. Kim, H. Y. Park, S. K. Ha, S. H. Lee, S. J. Na, and T. D. Choi. 2013. Method of separating and gathering capsaicinoid and carotenoid from red pepper (Capsicium annuum l.) using supercritical fluid extraction. Patent No. KR20130076102, filed December 28, 2011, and issued July 8, 2013.
- Choubert, G., and M. Baccaunaud. 2010. Effect of moist or dry heat cooking procedures on carotenoid retention and colour of fillets of rainbow trout (Oncorhynchus mykiss) fed astaxanthin or canthaxanthin. Food Chemistry 119 (1):265–9. doi: 10.1016/j.foodchem.2009.06.023.
- Choung, M. G., J. D. Lim, Y. S. Hwang, M. S. Lee, J. H. Lee, and Y. G. Kim. 2014. Extraction method of lutein from green tea. Patent No. KR20140082601, filed April 24, 2014, and issued July 12, 2014.
- Christiansen, R., O. Lie, and O. J. Torrissen. 1995. Growth and survival of Atlantic salmon, Salmo salar L., fed different dietary levels of astaxanthin. First-feeding fry. Aquaculture Nutrition 1 (3):189–1 98. doi: 10.1111/j.1365-2095.1995.tb00043.x.
- Christie, J. M. 2007. Phototropin Blue-Light Receptors. Annual Review of Plant Biology 58:21–45. doi: 10.1146/annurev.arplant.58.032806.103951.
- Chu, F. L., L. Pirastru, R. Popovic, and L. Sleno. 2011. Carotenogenesis Up-regulation in Scenedesmus sp. using a Targeted Metabolomics Approach by Liquid chromatography-high-resolution mass spectrometry . Journal of Agricultural and Food Chemistry 59 (7):3004–13. doi: 10.1021/jf105005q.
- Chuyen, H. V., P. D. Roach, J. B. Golding, S. E. Parks, and M. H. Nguyen. 2019. Ultrasound-assisted extraction of GAC peel: An optimization of extraction conditions for recovering carotenoids and antioxidant capacity. Processes 8 (1):8. doi: 10.3390/pr8010008.
- Ciriminna, R., A. Fidalgo, F. Meneguzzo, L. M. Ilharco, and M. Pagliaro. 2016. Lycopene: Emerging Production Methods and Applications of a Valued Carotenoid. ACS Sustainable Chemistry & Engineering 4 (3):643–50. doi: 10.1021/acssuschemeng.5b01516.
- Commission Regulation (EU) No 257/2010 of 25 March 2010 setting up a programme for the re-evaluation of approved food additives in accordance with Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives. Official Journal of the European Union L80/19.
- Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. www.data.europa.eu/eli/reg/2012/231/2019-10-23.
- Conklin, A. I., N. G. Forouhi, M. Suhrcke, P. Surtees, N. J. Wareham, and P. Monsivais. 2014. Variety more than quantity of fruit and vegetable intake varies by socioeconomic status and financial hardship. Findings from older adults in the EPIC cohort. Appetite 83:248–55. doi: 10.1016/j.appet.2014.08.038.
- Coyago-Cruz, E., M. Corell, A. Moriana, D. Hernanz, C. M. Stinco, and A. J. Meléndez-Martínez. 2017. Effect of the fruit position on the cluster on fruit quality, carotenoids, phenolics and sugars in cherry tomatoes (Solanum lycopersicum L.). Food Research International (Ottawa, Ont.) 100 (Pt 1):804–13. doi: 10.1016/j.foodres.2017.08.002.
- Coyago-Cruz, E., M. Corell, A. Moriana, D. Hernanz, A. M. Benítez-González, C. M. Stinco, and A. J. Meléndez-Martínez. 2018. Antioxidants (carotenoids and phenolics) profile of cherry tomatoes as influenced by deficit irrigation, ripening and cluster. Food Chemistry 240:870–84. doi: 10.1016/j.foodchem.2017.08.028.
- Coyago-Cruz, E., M. Corell, C. M. Stinco, D. Hernanz, A. Moriana, and A. J. Meléndez-Martínez. 2017. Effect of regulated deficit irrigation on quality parameters, carotenoids and phenolics of diverse tomato varieties (Solanum lycopersicum L.). Food Research International (Ottawa, Ont.) 96:72–83. doi: 10.1016/j.foodres.2017.03.026.
- Craver, J. K., J. R. Gerovac, R. G. Lopez, and D. A. Kopsell. 2017. Light intensity and light quality from sole-source light-emitting diodes impact phytochemical concentrations within Brassica microgreens. Journal of the American Society for Horticultural Science 142 (1):3–12. doi: 10.21273/JASHS03830-16.
- Curran-Celentano, J., B. R. Hammond, Jr, T. A. Ciulla, D. A. Cooper, L. M. Pratt, and R. B. Danis. 2001. Relation between dietary intake, serum concentrations, and retinal concentrations of lutein and zeaxanthin in adults in a Midwest population. The American Journal of Clinical Nutrition 74 (6):796–802. doi: 10.1093/ajcn/74.6.796.
- Daley, C. A., A. Abbott, P. S. Doyle, G. A. Nader, and S. Larson. 2010. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutrition Journal 9 (1):10. doi: 10.1186/1475-2891-9-10.
- Darwish, W. S., Y. Ikenaka, A. E. Morshdy, K. I. Eldesoky, S. Nakayama, H. Mizukawa, and M. Ishizuka. 2016. β-Carotene and retinol contents in the meat of herbivorous ungulates with a special reference to their public health importance. The Journal of Veterinary Medical Science 78 (2):351–4. doi: 10.1292/jvms.15-0287.
- Darwish, W. S., Y. Ikenaka, M. Ohno, E. A. Eldaly, and M. Ishizuka. 2010. Carotenoids as regulators for inter-species difference in Cytochrome P450 1A expression and activity in ungulates and rats. Food and Chemical Toxicology 48 (11):3201–8. doi: 10.1016/j.fct.2010.08.022.
- Daury, M. C., and M. A. Juillerat. 2000. Carotenoid and other anti-oxidants extraction. Patent No. WO0069284.
- Dawson, T. L. 2008. It must be green: Meeting society’s environmental concerns. Coloration Technology 124 (2):67–78. doi: 10.1111/j.1478-4408.2008.00124.x.
- De Carbonnel, M., P. Davis, M. R. G. Roelfsema, S. I. Inoue, I. Schepens, P. Lariguet, M. Geisler, K. I. Shimazaki, R. Hangarter, and C. Fankhauser. 2010. The Arabidopsis phytochrome kinase substrate2 protein is a phototropin signaling element that regulates leaf flattening and leaf positioning. Plant Physiology 152 (3):1391–405. doi: 10.1104/pp.109.150441.
- De Carvalho, C. C., and M. J. Caramujo. 2017. Carotenoids in aquatic ecosystems and aquaculture: A colorful business with implications for human health. Frontiers in Marine Science 4:93. doi: 10.3389/fmars.2017.00093.
- De la Parra, C., S. O. Serna Saldivar, and R. H. Liu. 2007. Effect of processing on the phytochemical profiles and antioxidant activity of corn for production of masa, tortillas, and tortilla chips. Journal of Agricultural and Food Chemistry 55 (10):4177–83. doi: 10.1021/jf063487p.
- De Oliveira, G. P., and D. B. Rodríguez-Amaya. 2007. Processed and prepared corn products as sources of lutein and zeaxanthin: Compositional variation in the food chain. Journal of Food Science 72 (1):S079–S085. doi: 10.1111/j.1750-3841.2006.00235.x.
- De Rosso, V. V., and A. Z. Mercadante. 2007. Identification and quantification of carotenoids, by HPLC-PDA-MS/MS, from Amazonian fruits. Journal of Agricultural and Food Chemistry 55 (13):5062–72. doi: 10.1021/jf0705421.
- Delgado-Pelayo, R., L. Gallardo-Guerrero, and D. Hornero-Méndez. 2016. Carotenoid composition of strawberry tree (Arbutus unedo L.) fruits. Food Chemistry 199:165–75. doi: 10.1016/j.foodchem.2015.11.135.
- Delgado-Pelayo, R., and D. Hornero-Méndez. 2012. Identification and quantitative analysis of carotenoids and their esters from Sarsaparilla (Smilax aspera L.) berries. Journal of Agricultural and Food Chemistry 60 (33):8225–32. doi: 10.1021/jf302719g.
- Delgado-Vargas, F., A. R. Jiménez, and O. Paredes-López. 2000. Natural Pigments: Carotenoids, Anthocyanins, and betalains-characteristics, biosynthesis, processing, and stability. Critical Reviews in Food Science and Nutrition 40 (3):173–289. doi: 10.1080/10408690091189257.
- Deng, L., Z. Yuan, J. Xie, S. Yao, and K. Zeng. 2017. Sensitivity to ethephon degreening treatment is altered by blue LED light irradiation in mandarin fruit. Journal of Agricultural and Food Chemistry 65 (30):6158–68. doi: 10.1021/acs.jafc.7b01703.
- Descalzo, A. M., E. M. Insani, A. Biolatto, A. M. Sancho, P. T. García, N. A. Pensel, and J. A. Josifovich. 2005. Influence of pasture or grain-based diets supplemented with vitamin E on antioxidant/oxidative balance of Argentine beef. Meat Science 70 (1):35–44. doi: 10.1016/j.meatsci.2004.11.018.
- Dettmer, K., and B. D. Hammock. 2004. Metabolomics—A new exciting field within the “omics” sciences. Environmental Health Perspectives 112 (7):A396–A397. doi: 10.1289/ehp.112-1241997.
- Dhakal, R., and K. H. Baek. 2014. Short period irradiation of single blue wavelength light extends the storage period of mature green tomatoes. Postharvest Biology and Technology 90:73–7. doi: 10.1016/j.postharvbio.2013.12.007.
- Dian, P. H. M., D. Andueza, C. M. P. Barbosa, S. Amoureux, M. Jestin, P. C. F. Carvalho, S. Amoureux, M. Jestin, P. C. F. Carvalho, I. N. Prado, et al. 2007. Methodological developments in the use of visible reflectance spectroscopy for discriminating pasture-fed from concentrate-fed lamb carcasses. Animal 1 (8):1198–08. doi: 10.1017/S175173110700047X.
- Dian, P. H. M., B. Chauveau-Duriot, I. N. Prado, and S. Prache. 2007. A dose-response study relating the concentration of carotenoid pigments in blood and reflectance spectrum characteristics of fat to carotenoid intake level in sheep. Journal of Animal Science 85 (11):3054–61. doi: 10.2527/jas.2006-477.
- Dias, M. G., M. F. G. Camões, and L. Oliveira. 2009. Carotenoids in traditional Portuguese fruits and vegetables. Food Chemistry 113 (3):808–15. doi: 10.1016/j.foodchem.2008.08.002.
- Dias, M. G., M. F. G. Camões, and L. Oliveira. 2014. Carotenoid stability in fruits, vegetables and working standards—Effect of storage temperature and time. Food Chemistry 156:37–41. doi: 10.1016/j.foodchem.2014.01.050.
- Dias, M. G., B. Olmedilla-Alonso, D. Hornero-Méndez, A. Z. Mercadante, C. Osorio, L. Vargas-Murga, and A. J. Meléndez-Martínez. 2018. Comprehensive Database of Carotenoid Contents in Ibero-American Foods. A Valuable Tool in the Context of Functional Foods and the Establishment of Recommended Intakes of Bioactives. Journal of Agricultural and Food Chemistry 66 (20):5055–107. doi: 10.1021/acs.jafc.7b06148.
- Diep, T. T., C. Pook, E. C. Rush, and M. J. Y. Yoo. 2020. Quantification of carotenoids, α-tocopherol, and ascorbic acid in amber, mulligan, and laird’s large cultivars of New Zealand tamarillos (Solanum betaceum Cav.). Foods 9 (6):769–16. doi: 10.3390/foods9060769.
- Dineshkumar, R., and R. Sen. 2020. A sustainable perspective of microalgal biorefinery for co-production and recovery of high-value carotenoid and biofuel with CO2 valorization. Biofuels, Bioproducts and Biorefining 14 (4):879–19. doi: 10.1002/bbb.2107.
- Diprat, A. B., R. C. Silveira Thys, E. Rodrigues, and R. Rech. 2020. Chlorella sorokiniana: A new alternative source of carotenoids and proteins for gluten-free bread. LWT - Food Science and Technology 134:109974. doi: 10.1016/j.lwt.2020.109974.
- Djuric, Z., J. Ren, J. Blythe, G. VanLoon, and A. Sen. 2009. A Mediterranean dietary intervention in healthy American women changes plasma carotenoids and fatty acids in distinct clusters. Nutrition Research (New York, N.Y.) 29 (3):156–63. doi: 10.1016/j.nutres.2009.03.001.
- Döker, O., U. Salgın, İ. Şanal, Ü. Mehmetoğlu, and A. Çalımlı. 2004. Modeling of extraction of β-carotene from apricot bagasse using supercritical CO2 in packed bed extractor. The Journal of Supercritical Fluids 28 (1):11–9. doi: 10.1016/S0896-8446(03)00006-8.
- Du, W.-X., R. J. Avena-Bustillos, A. P. Breksa, III, and T. H. McHugh. 2012. Effect of UV-B light and different cutting styles on antioxidant enhancement of commercial fresh-cut carrot products. Food Chemistry 134 (4):1862–9. doi: 10.1016/j.foodchem.2012.03.097.
- Du, X., H. Ni, G. Huang, Y. Yang, L. Li, A. Xiao, and H. Cai. 2014. Method of separating and purifying astaxanthin from Phaffia rhodozyma. Patent No. CN103848769, filed February 21, 2014, and issued June 11, 2014.
- Dufossé, L. 2006. Microbial production of food grade pigments. Food Technology and Biotechnology 44 (3):313–23.
- Dugo, P., M. Herrero, D. Giuffrida, C. Ragonese, G. Dugo, and L. Mondello. 2008. Analysis of native carotenoid composition in orange juice using C30 columns in tandem. Journal of Separation Science 31 (12):2151–60. doi: 10.1002/jssc.200800073.
- Dugo, P., M. Herrero, T. Kumm, D. Giuffrida, G. Dugo, and L. Mondello. 2008. Comprehensive normal-phase x reversed-phase liquid chromatography coupled to photodiode array and mass spectrometry detection for the analysis of free carotenoids and carotenoid esters from mandarin . Journal of Chromatography. A 1189 (1–2):196–206. doi: 10.1016/j.chroma.2007.11.116.
- Dunne, P. G., F. P. O’Mara, F. J. Monahan, and A. P. Moloney. 2006. Changes in colour characteristics andvpigmentation of subcutaneous adipose tissue and M. longissimus dorsi of heifers fed grass, grassvsilage or concentrate-based diets. Meat Science 74 (2):231–41. doi: 10.1016/j.meatsci.2006.02.003.
- Dzomeku, B. M., J. P. Wald, J. N. Wünsche, D. Nohr, and H. K. Biesalski. 2020. Climate Change Enhanced Carotenoid Pro-Vitamin A Levels of Selected Plantain Cultivars. Plants 9 (4):541. doi: 10.3390/plants9040541.
- Eggersdorfer, M., and A. Wyss. 2018. Carotenoids in human nutrition and health. Archives of Biochemistry and Biophysics 652:18–26. doi: 10.1016/j.abb.2018.06.001.
- EFSA. 2014. Guidance on the EU Menu methodology, Parma Italy. EFSA Journal 12 (12):3944.
- EFSA. 2015. The food classification and description system FoodEx2 (revision 2). Technical Report. .
- EFSA ANS Panel. 2016. Scientific opinion on the safety of annatto extracts (E 160b) as a food additive. EFSA Journal 4 (8):4544.
- Eh, A. L.-S., and S.-G. Teoh. 2012. Novel modified ultrasonication technique for the extraction of lycopene from tomatoes. Ultrasonics Sonochemistry 19 (1):151–9. doi: 10.1016/j.ultsonch.2011.05.019.
- Eismann, A. I., R. Perpetuo Reis, A. Ferreira da Silva, and D. Negrão Cavalcanti. 2020. Ulva spp. carotenoids: Responses to environmental conditions. Algal Research 48:101916. doi: 10.1016/j.algal.2020.101916.
- Elgersma, A., K. Søegaard, and S. K. Jensen. 2013. Fatty acids, α-tocopherol, β-carotene, and lutein contents in forage legumes, forbs, and a grass-clover mixture. Journal of Agricultural and Food Chemistry 61 (49):11913–20. doi: 10.1021/jf403195v.
- Elgersma, A., K. Søegaard, and S. K. Jensen. 2015. Interrelations between herbage yield, α-tocopherol, β-carotene, lutein, protein, and fiber in non-leguminous forbs, forage legumes, and a grass-clover mixture as affected by harvest date. Journal of Agricultural and Food Chemistry 63 (2):406–14. doi: 10.1021/jf503658n.
- Elik, A., D. K. Yanık, and F. Göğüş. 2020. Microwave-assisted extraction of carotenoids from carrot juice processing waste using flaxseed oil as a solvent. LWT - Food Science and Technology 123:109100. doi: 10.1016/j.lwt.2020.109100.
- El-Sohemy, A., A. Baylin, E. Kabagambe, A. Ascherio, D. Spiegelman, and H. Campos. 2002. Individual carotenoid concentrations in adipose tissue and plasma as biomarkers of dietary intake. The American Journal of Clinical Nutrition 76 (1):172–9. doi: 10.1093/ajcn/76.1.172.
- Englmaierová, M., M. Skřivan, and I. Bubancová. 2013. A comparison of lutein, spray-dried Chlorella, and synthetic carotenoids effects on yolk colour, oxidative stability, and reproductive performance of laying hens. Czech Journal of Animal Science 58 (No. 9):412–9. doi: 10.17221/6941-CJAS.
- Esteban, R., J. F. Moran, J. M. Becerril, and J. I. García-Plazaola. 2015. Versatility of carotenoids: An integrated view on diversity, evolution, functional roles and environmental interactions. Environmental and Experimental Botany 119:63–75. doi: 10.1016/j.envexpbot.2015.04.009.
- Estévez-Santiago, R., B. Beltrán-de-Miguel, and B. A. Olmedilla-Alonso. 2016. Assessment of dietary lutein, zeaxanthin and lycopene intakes and sources in the Spanish Survey of Dietary Intake (2009–2010). International Journal of Food Sciences and Nutrition 67 (3):305–13. doi: 10.3109/09637486.2016.1147020.
- Estrella De Castro, A., A. J. Collados De La Vieja, E. Bernasconi, M. Esteban Morales, and E. Gonzalez De Prado. 2001. Process for producing lycopen. Patent No. WO0112832, filed July 21, 2000, and issued February 22, 2001.
- Estrella De Castro, A., N. Fraile Yecora, M. Oliver Ruiz, A. Munoz, J. F. Lopez Ortiz, and W. Cabri. 2004. Method of obtaining novel lutein-based formulations. Patent No. EP1460060, filed December 20, 2002, and issued September 22, 2004.
- European Union Register of Feed Additives. 2018. European Union Register of Feed Additives persuant to Regulation (EC) No 1831/2003. Annex I: List of additives, Eddition 1/2018 (260). Directorate-General for Health and Food Safety, European Commission, Brussels.
- Fankhauser, C., and J. Chory. 1997. Light control of plant development. Annual Review of Cell and Developmental Biology 13:203–29. doi: 10.1146/annurev.cellbio.13.1.203.
- Félix-Valenzuela, L., I. Higuera-Ciapara, F. Goycoolea-Valencia, and W. Argüelles-Monal. 2001. Supercritical CO2/ethanol extraction of astaxanthin from blue crab (Callinectes sapidus) shell waste. Journal of Food Process Engineering 24 (2):101–12.
- Ferrari, D., A. Aldini, S. Cuccolini, D. Ferrari, A. Aldini, and S. Cuccolini. 2013. Carotenoid extraction from plant material. Patent No. US2013085309, filed September 30, 2011, and issued April 4, 2013.
- Ferreira de Faria, A., P. N. Hasegawa, E. Alves Chagas, R. Pio, E. Purgatto, and A. Z. Mercadante. 2009. Cultivar influence on carotenoid composition of loquats from Brazil. Journal of Food Composition and Analysis 22 (3):196–203. doi: 10.1016/j.jfca.2008.10.014.
- Ficco, D. B. M., A. M. Mastrangelo, D. Trono, G. M. Borrelli, P. De Vita, C. Fares, R. Beleggia, C. Platani, and R. Papa. 2014. The colours of durum wheat: A review. Crop and Pasture Science 65 (1):1–15. doi: 10.1071/CP13293.
- Folta, K. M., and K. S. Childers. 2008. Light as a growth regulator: Controlling plant biology with narrow-bandwidth solid-state lighting systems. HortScience 43 (7):1957–64. doi: 10.21273/HORTSCI.43.7.1957.
- Folta, K. M., L. L. Koss, R. McMorrow, H. H. Kim, J. D. Kenitz, R. Wheeler, and J. C. Sager. 2005. Design and fabrication of adjustable red-green-blue LED light arrays for plant research. BMC Plant Biology 5 (1):17. doi: 10.1186/1471-2229-5-17.
- Folta, K. M., and S. A. Maruhnich. 2007. Green light: A signal to slow down or stop. Journal of Experimental Botany 58 (12):3099–111. doi: 10.1093/jxb/erm130.
- Fox, D. L. 1979. Biochromy, natural coloration of living things. Berkeley, CA: University of California Press.
- Franke, S., K. Fröhlich, S. Werner, V. Böhm, and F. Schöne. 2010. Analysis of carotenoids and vitamin E in selected oilseeds, press cakes and oils. European Journal of Lipid Science and Technology 112 (10):1122–9. doi: 10.1002/ejlt.200900251.
- Fraser, G. E., K. Jaceldo-Siegl, S. M. Henning, J. Fan, S. F. Knutsen, E. H. Haddad, J. Sabaté, W. L. Beeson, and H. Bennett. 2016. Biomarkers of dietary intake are correlated with corresponding measures from repeated dietary recalls and food-frequency questionnaires in the Adventist Health Study-2. The Journal of Nutrition 146 (3):586–94. doi: 10.3945/jn.115.225508.
- Fraser, P. D., E. M. Enfissi, M. Goodfellow, T. Eguchi, and P. M. Bramley. 2007. Metabolite profiling of plant carotenoids using the matrix-assisted laser desorption ionization time-of-flight mass spectrometry. The Plant Journal 49 (3):552–64. doi: 10.1111/j.1365-313X.2006.02949.x.
- Fratianni, A., T. Di Criscio, R. Mignogna, and G. Panfili. 2012. Carotenoids, tocols and retinols evolution during egg pasta–making processes. Food Chemistry 131 (2):590–5. doi: 10.1016/j.foodchem.2011.09.034.
- Galaup, P., N. Sutthiwong, M. N. Leclercq-Perlat, A. Valla, Y. Caro, M. Fouillaud, F. Guérard, and L. Dufossé. 2015. First isolation of Brevibacterium sp. pigments in the rind of an industrial red-smear-ripened soft cheese. International Journal of Dairy Technology 68 (1):144–7. doi: 10.1111/1471-0307.12211.
- Gangadhar, B. H., R. K. Mishra, G. Pandian, and S. W. Park. 2012. Comparative study of color, pungency, and biochemical composition in chili pepper (Capsicum annuum) under different light-emitting diode treatments. HortScience 47 (12):1729–35. doi: 10.21273/HORTSCI.47.12.1729.
- García-Alonso, F. J., S. Bravo, J. Casas, D. Pérez-Conesa, K. Jacob, and M. J. Periago. 2009. Changes in antioxidant compounds during the shelf life of commercial tomato juices in different packaging materials. Journal of Agricultural and Food Chemistry 57 (15):6815–22. doi: 10.1021/jf900877c.
- Garcia-Mendoza, M. P., J. T. Paula, L. C. Paviani, F. A. Cabral, and H. A. Martinez-Correa. 2015. Extracts from mango peel by-product obtained by supercritical CO2 and pressurized solvent processes. LWT - Food Science and Technology 62 (1):131–7. doi: 10.1016/j.lwt.2015.01.026.
- García-Romero, J., R. Ginés, M. S. Izquierdo, R. Haroun, R. Badilla, and L. Robaina. 2014. Effect of dietary substitution of fish meal for marine crab and echinoderm meals on growth performance, ammonia excretion, skin colour, and flesh quality and oxidation of red porgy (Pagrus pagrus). Aquaculture 422–423:239–48. doi: 10.1016/j.aquaculture.2013.11.024.
- Garnett, K. M., L. H. Guerra-Santos, and D. L. Gierhart. 2003. Zeaxanthin formulations for human ingestion. Patent No. US2003108598, filed December 17, 2002, and issued June 12, 2003.
- Gay, L., D. S. Kronfeld, A. Grimsley-Cook, J. Dascanio, A. Ordakowski-Burk, R. K. Splan, E. A. Dunnington, and D. J. Sklan. 2004. Retinol, β-carotene and β-tocopherol concentrations in mare and foal plasma and in colostrum. Journal of Equine Veterinary Science 24 (3):115–20. doi: 10.1016/j.jevs.2004.02.005.
- Gayosso-García Sancho, L. E., E. M. Yahia, and G. A. González-Aguilar. 2011. Identification and quantification of phenols, carotenoids, and vitamin C from papaya (Carica papaya L., cv. Maradol) fruit determined by HPLC-DAD-MS/MS-ESI. Food Research International 44 (5):1284–91. doi: 10.1016/j.foodres.2010.12.001.
- Gellenbeck, K. W. 1998. Dry carotenoid-oil powder and process for making same. Patent No. US5827539, filed December 28, 1995, issued October 27, 1998.
- General Standard for Food Additives. 2016. CODEX STAN 192–1995.
- George, S. M., F. E. Thompson, D. Midthune, A. F. Subar, D. Berrigan, A. Schatzkin, and N. Potischman. 2012. Strength of the relationships between three self-reported dietary intake instruments and serum carotenoids: The Observing Energy and Protein Nutrition (OPEN) Study. Public Health Nutrition 15 (6):1000–7. doi: 10.1017/S1368980011003272.
- Georgiovic, G. R., P. O. Viktorivna, N. L. Leonidivna, and N. M. Valentinovic. 2014. A method of obtaining of carotenoid complex from spirulina biomass. Patent No. UA94198, filed February 5, 2014, and issued November 10, 2014.
- Gibbons, H., A. O'Gorman, and L. Brennan. 2015. Metabolomics as a tool in nutritional research. Current Opinion in Lipidology 26 (1):30–4. doi: 10.1097/MOL.0000000000000140.
- Girard, P., C. E. J. Javelot, and B. D. V. Vladescu. 2001. Phaffia rhodozyma mutants, process for the production of beta-caroten and use of a beta-caroten rich biomass. Patent No. GR3036025, filed June 13, 2001, issued September 28, 2001.
- Giuffrida, D., F. Cacciola, P. Mapelli-Brahm, C. M. Stinco, P. Dugo, M. Oteri, L. Mondello, and A. J. Meléndez-Martínez. 2019. Free carotenoids and carotenoids esters composition in Spanish orange and mandarin juices from diverse varieties. Food Chemistry 300:125139. doi: 10.1016/j.foodchem.2019.125139.
- Giuffrida, D., A. Pintea, P. Dugo, G. Torre, R. M. Pop, and L. Mondello. 2012. Determination of carotenoids and their esters in fruits of sea buckthorn (Hippophae rhamnoides L.) by HPLC-DAD-APCI-MS. Phytochemical Analysis: PCA 23 (3):267–273. doi: 10.1002/pca.1353.
- Giuffrida, D., N. Sutthiwong, P. Dugo, P. Donato, F. Cacciola, E. Girard-Valenciennes, Y. Le Mao, C. Monnet, M. Fouillaud, Y. Caro, et al. 2016. Characterisation of the C50 carotenoids produced by strains of the cheese-ripening bacterium Arthrobacter arilaitensis. International Dairy Journal 55:10–16. doi: 10.1016/j.idairyj.2015.11.005.
- Giuffrida, D., M. Zoccali, S. V. Giofrè, P. Dugo, and L. Mondello. 2017. Apocarotenoids determination in Capsicum chinense Jacq. cv Habanero, by supercritical fluid chromatography-triple-quadrupole/mass spectrometry. Food Chemistry 231:316–323. doi: 10.1016/j.foodchem.2017.03.145.
- Golzar Adabi, S. H., M. A. Kamali, J. Davoudi, R. G. Cooper, and A. Hajbabaei. 2010. Quantification of lutein in egg following feeding hens with a lutein supplement and quantification of lutein in human plasma after consumption of lutein enriched eggs. Archiv Für Geflügelkunde 74 (3):158–163.
- Gómez, F. A. R., G. R. Peña, A. D. Cota, C. A. M. Rubio, and N. Y. V. Rodríguez. 2016. Extraction of carotenoids by biotechnological and mechanical methods for the production of virgin oleoresins. Patent No. MX2015000663, filed December 19, 2014, issued June 20, 2016.
- González, M., E. Castaño, E. Avila, and E. González de Mejía. 1999. Effect of capsaicin from red pepper (Capsicum sp) on the deposition of carotenoids in egg yolk. Journal of the Science of Food and Agriculture 79 (13):1904–08. doi: 10.1002/(SICI)1097-0010(199910)79:13<1904::AID-JSFA452>3.0.CO;2-S.
- Gorocica-Buenfil, M. A., F. L. Fluharty, T. Bohn, S. J. Schwartz, and S. C. Loerch. 2007. Effect of low vitamin A diets with high-moisture or dry corn on marbling and adipose tissue fatty acid composition of beef steers. Journal of Animal Science 85 (12):3355–66. doi: 10.2527/jas.2007-0172.
- Granado, F., S. Blázquez, and B. Olmedilla. 2007. Changes in carotenoid intake from fruit and vegetables in the Spanish population over the period 1964–2004. Public Health Nutrition 10 (10):1018–1023. doi: 10.1017/S1368980007662314.
- Granado, F., B. Olmedilla, I. Blanco, and E. Rojas-Didalgo. 1996. Major fruit and vegetable contributors to the main serum carotenoids in the Spanish diet. European Journal of Clinical Nutrition 50 (4):246–250.
- Grass, H., and A. Hitzfeld. 2016. New red color for edible coatings. Patent No. TW201642764, filed March 24, 2016, issued December 16, 2016.
- Green, A. S., and A. J. Fascetti. 2016. Meeting the vitamin A requirement: The efficacy and importance of β-carotene in animal species. The Scientific World Journal 2016:1–22. doi: 10.1155/2016/7393620.
- Greiwe-Crandell, K. M., D. S. Kronfeld, L. S. Gay, D. Sklan, W. Tiegs, and P. A. Harris. 1997. Vitamin A repletion in thoroughbred mares with retinyl palmitate or β-carotene. Journal of Animal Science 75 (10):2684–90. doi: 10.2527/1997.75102684x.
- Guan, Y., L. Zhou, J. Bi, J. Yi, X. Liu, Q. Chen, X. Wu, and M. Zhou. 2016. Change of microbial and quality attributes of mango juice treated by high pressure homogenization combined with moderate inlet temperatures during storage. Innovative Food Science & Emerging Technologies 36:320–329. doi: 10.1016/j.ifset.2016.07.009.
- Guo, J., S. Suo, and B. Wang. 2017. Extraction process of suaeda salsa seed carotenoid. Patent No. CN106727750, filed November 10, 2016, and issued May 31, 2017.
- Guo, X., X. Hao, J. M. Zheng, C. Little, and S. Khosla. 2016. Response of greenhouse mini-cucumber to different vertical spectra of LED lighting under overhead high pressure sodium and plasma lighting. Acta Horticulturae 1170 (1134):87–10. doi: 10.17660/ActaHortic.2016.1134.12.
- Gupta, S. D., and A. Dutta Agarwal. 2017. Light emitting diodes for agriculture. Singapore: Springer.
- Guyomarc'h, F., A. Binet, and L. Dufossé. 2000. Production of carotenoids by Brevibacterium linens: Variation among strains, kinetic aspects and HPLC profiles. Journal of Industrial Microbiology and Biotechnology 24 (1):64–70. doi: 10.1038/sj.jim.2900761.
- Haard, N. F. 1992. Biochemistry and chemistry of color and color change in seafood. In Advances in seafood biochemistry: Composition and quality, eds. G. J. Flick and R.E. Martin, 305–60. Lancaster and Basel: Technomic.
- Hai, Y., L. Shuo, Z. Bin, X. Qianqian, L. Gang, and Z. Mingzhong. 2008. Method for extracting and purifying xanthophyl from chlorella algae powder. Patent No. CN101130513, filed August 24, 2007, and issued February 24, 2008.
- Han, G. D., and H. J. Jeong. 2016. Method for extracting carotenoid from fermented persimmon sludge. Patent No. KR20160024052, filed August 22, 2014, issued March 4, 2016.
- Hao, X., C. Little, J. M. Zheng, and R. Cao. 2016. Far-red LEDs improve fruit production in greenhouse tomato grown under high-pressure sodium lighting. Acta Horticulturae 1134 (1134):95–102. doi: 10.17660/ActaHortic.2016.1134.13.
- Hasperue, J. H., L. M. Rodoni, L. M. Guardianelli, A. R. Chaves, and G. A. Martinez. 2016. Use of LED light for Brussels sprouts postharvest conservation. Scientia Horticulturae 213:281–286. doi: 10.1016/j.scienta.2016.11.004.
- He, M., and J. Huang. 2017. Supercritical carbon dioxide fluid extraction method of astaxanthin of genetically-engineered tomato fruit. Patent No. CN107235881, filed June 15, 2017, and issued October 10, 2017.
- Hegeman, A. D. 2010. Plant metabolomics-meeting the analytical challenges of comprehensive metabolite analysis. Briefings in Functional Genomics 9 (2):139–148. doi: 10.1093/bfgp/elp053.
- Hempel, J., C. N. Schädle, J. Sprenger, A. Heller, R. Carle, and R. M. Schweiggert. 2017. Ultrastructural deposition forms and bioaccessibility of carotenoids and carotenoid esters from goji berries (Lycium barbarum L.). Food Chemistry 218:525–533. doi: 10.1016/j.foodchem.2016.09.065.
- Hendriks, M. M., F. A. van Eeuwijk, R. H. Jellema, J. A. Westerhuis, T. H. Reijmers, H. C. Hoefsloot, and A. K. Smilde. 2011. Data-processing strategies for metabolomics studies. TrAC Trends in Analytical Chemistry 30 (10):1685–1698. doi: 10.1016/j.trac.2011.04.019.
- Herrero, M., F. Cacciola, P. Donato, D. Giuffrida, G. Dugo, P. Dugo, and L. Mondello. 2008. Serial coupled columns reversed-phase separations in high-performance liquid chromatography. Tool for analysis of complex real samples. Journal of Chromatography. A 1188 (2):208–215. doi: 10.1016/j.chroma.2008.02.039.
- Heuberger, H., U. Praeger, M. Georgi, G. Schirrmacher, J. Graßmann, and W. H. Schnitzler. 2004. Precision stressing by UV-B radiation to improve quality of spinach under protected cultivation. Acta Horticulturae 659 (659):201–206. In ( doi: 10.17660/ActaHortic.2004.659.25.
- Hidalgo, A., A. Brandolini, and C. Pompei. 2010. Carotenoids evolution during pasta, bread and water biscuit preparation from wheat flours. Food Chemistry 121 (3):746–751. doi: 10.1016/j.foodchem.2010.01.034.
- Hiranvarachat, B., and S. Devahastin. 2014. Enhancement of microwave-assisted extraction via intermittent radiation: Extraction of carotenoids from carrot peels. Journal of Food Engineering 126:17–26. doi: 10.1016/j.jfoodeng.2013.10.024.
- Hisano, H., J. L. Pilecco, and J. A. Ferreira de Lara. 2016. Corn gluten meal in pacu Piaractus mesopotamicus diets: Effects on growth, haematology, and meat quality. Aquaculture International 24 (4):1049–60. doi: 10.1007/s10499-016-9970-7.
- Hoffmann, A. M., G. Noga, and M. Hunsche. 2016. Alternating high and low intensity of blue light affects PSII photochemistry and raises the contents of carotenoids and anthocyanins in pepper leaves. Plant Growth Regulation 79 (3):275–285. doi: 10.1007/s10725-015-0132-0.
- Holden, J. M., A. L. Eldridge, G. R. Beecher, I. M. Buzzard, S. Bhagwat, C. S. Davis, L. W. Douglass, S. Gebhardt, D. Haytowitz, and S. Schakel. 1999. Carotenoid content of US foods: An update of the database. Journal of Food Composition and Analysis 12 (3):169–196. doi: 10.1006/jfca.1999.0827.
- Honest, K. N., H. W. Zhang, and L. Zhang. 2011. Lycopene: Isomerization effects on bioavailability and bioactivity properties. Food Reviews International 27 (3):248–58. doi: 10.1080/87559129.2011.563392.
- Hornero-Méndez, D. 2019. Occurrence of carotenoid esters in foods. In Carotenoid esters in foods: Physical, chemical and biological properties, ed. A. Z. Mercadante, 181–284. London, UK: Royal Society of Chemistry.
- Hornero-Méndez, D., and M. I. Mínguez-Mosquera. 2000a. Xanthophyll esterification accompanying carotenoid overaccumulation in chromoplast of Capsicum annuum ripening fruits is a constitutive process and useful for ripeness index. Journal of Agricultural and Food Chemistry 48 (5):1617–22. doi: 10.1021/jf9912046.
- Hornero-Méndez, D., and M. I. Mínguez-Mosquera. 2000b. Carotenoid pigments in Rosa mosqueta hips, an alternative carotenoid source for foods. Journal of Agricultural and Food Chemistry 48 (3):825–828. doi: 10.1021/jf991136n.
- Hu, B., M. Ferrell, C. E. Lim, and D. A. Davis. 2012. Evaluation of traditional diet and corn gluten feed substituted alternative diet for pond-raised hybrid catfish on production and xanthophyll level. Aquaculture 354–355:22–26. doi: 10.1016/j.aquaculture.2012.04.038.
- Huang, Z., M. J. Yang, Q. Ma, and S. F. Liu. 2016. Supercritical CO2 extraction of Chinese lantern: Experimental and OEC modeling. Separation and Purification Technology 159:23–34. doi: 10.1016/j.seppur.2015.12.051.
- Hulshof, P. J., T. van Roekel-Jansen, P. van de Bovenkamp, and C. E. West. 2006. Variation in retinol and carotenoid content of milk and milk products in The Netherlands. Journal of Food Composition and Analysis 19 (1):67–75. doi: 10.1016/j.jfca.2005.04.005.
- Inomata, H., N. Sato, S. Mori, and Y. Suzuki. 2007. Method for producing carotenoid pigment. Patent No. JP2007046015, filed August 12, 2005, and issued February 22, 2007.
- Isabelle, M., B. L. Lee, M. T. Lim, W.-P. Koh, D. Huang, and C. N. Ong. 2010. Antioxidant activity and profiles of common fruits in Singapore. Food Chemistry 123 (1):77–84. doi: 10.1016/j.foodchem.2010.04.002.
- Ishida, B. K., M. H. Chapman, S. S. Randhava, and S. S. Randhava. 2009. Extraction of carotenoids from plant material. Patent No. US7572468, filed December 27, 2005, and issued August 11, 2009.
- Jenkins, G. I. 2014. Structure and function of the UV-B photoreceptor UVR8. Current Opinion in Structural Biology 29:52–57. doi: 10.1016/j.sbi.2014.09.004.
- Johnson, E. J. 2014. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutrition Reviews 72 (9):605–612. doi: 10.1111/nure.12133.
- Jones, S. T., K. J. Aryana, and J. N. Losso. 2005. Storage stability of lutein during ripening of cheddar cheese. Journal of Dairy Science 88 (5):1661–1670. doi: 10.3168/jds.S0022-0302(05)72838-1.
- Joseph, S., and A. Anandane. 2013. Process for production of high purity beta-carotene and lycopene crystals from fungal biomass. Patent No. US2013066124, filed May 16, 2011, and issued March 14, 2013.
- Jumaah, F., M. Plaza, V. Abrahamsson, C. Turner, and M. Sandahl. 2016. A fast and sensitive method for the separation of carotenoids using ultra-high performance supercritical fluid chromatography-mass spectrometry. Analytical and Bioanalytical Chemistry 408 (21):5883–94. doi: 10.1007/s00216-016-9707-5.
- Kagan, M., and S. Braun. 2003. Processes for extracting carotenoids and for preparing feed materials. Patent No. US2003044495, filed June 17, 2002, and issued March 6, 2003.
- Kalinowski, C. T., L. E. Robaina, H. Fernandez-Palacios, D. Schuchardt, and M. S. Izquierdo. 2005. Effect of different carotenoid sources and their dietary levels on red porgy (Pagrus pagrus) growth and skin colour. Aquaculture 244 (1–4):223–231. doi: 10.1016/j.aquaculture.2004.11.001.
- Kamiloglu, S., G. Toydemir, D. Boyacioglu, J. Beekwilder, R. D. Hall, and E. Capanoglu. 2016. A Review on the Effect of Drying on Antioxidant Potential of Fruits and Vegetables. Critical Reviews in Food Science and Nutrition 56 (sup1):S110–S129. doi: 10.1080/10408398.2015.1045969.
- Kanaya, K., K. Kinoshita, and M. Hirano. 2014. Method for producing carotenoid composition. Patent No. US2014179657, filed June 29, 2012, and issued June 26, 2014.
- Kanazawa, K., T. Hashimoto, S. Yoshida, P. Sungwon, and S. Fukuda. 2012. Short photoirradiation induces flavonoid synthesis and increases its production in postharvest vegetables. Journal of Agricultural and Food Chemistry 60 (17):4359–68. doi: 10.1021/jf300107s.
- Kanetani, T., and K. Kinoshita. 2016. Method for producing carotenoid composition. Patent No. JP2016032430, filed December 27, 2012, and issued March 10, 2016.
- Kanner, J., R. Granit, and A. Levy. 2005. Increasing bioavailability of carotenoids. Patent No. WO2005026739, filed September 13, 2004, and issued March 24, 2005.
- Karadas, F., E. Grammenidis, P. F. Surai, T. Acamovic, and N. H. C. Sparks. 2006. Effects of carotenoids from lucerne, marigold and tomato on egg yolk pigmentation and carotenoid composition. British Poultry Science 47 (5):561–566. doi: 10.1080/00071660600962976.
- Katsuyama, M., and T. Matsuno. 1988. Carotenoid and vitamin a*, and metabolism of carotenoids, beta-carotene, canthaxanthin, astaxanthin, zeaxanthin, lutein and tunaxanthin in tilapia tilapia nilotica. Comparative Biochemistry and Physiology, 90B (1):131–139.
- Khachik, F. 2005. Process for extraction and purification of lutein, zeaxanthin and rare carotenoids from marigold flowers and plants. Patent No. US2005038271, filed October 4, 2004, and issued February 17, 2005.
- Khachik, F., C. J. Spangler, J. C. Smith, L. M. Canfield, A. Steck, and H. Pfander. 1997. Identification, quantification, and relative concentrations of carotenoids and their metabolites in human milk and serum. Analytical Chemistry 69 (10):1873–1881. doi: 10.1021/ac961085i.
- Kim, D.-G., N.-H. Choi, H.-W. Lee, P.-I. Jang, B.-S. Kim, I.-H. Na, J.-S. Meang, C.-J. Kim, C.-T. Kim, Y.-J. Cho, et al. 2016. Method for efficiently extracting lycopene from plant. Patent No. WO2016204544, filed June 17, 2016, and issued December 22, 2016.
- Kirkhus, B., N. K. Afseth, G. I. A. Borge, S. Grimsby, C. Steppeler, A. Krona, and M. Langton. 2019. Increased release of carotenoids and delayed in vitro lipid digestion of high pressure homogenized tomato and pepper emulsions. Food Chemistry 285:282–289. doi: 10.1016/j.foodchem.2019.01.124.
- Klingenberg, A. 2005. Method for manufacturing a colour mixture for use in food products, pharmaceuticals and cosmetics and colour mixture obtained according to this method. Patent No. US2005084462, filed October 16, 2003, and issued April 21, 2005.
- Kljak, K., and D. Grbeša. 2015. Carotenoid content and antioxidant activity of hexane extracts from selected Croatian corn hybrids. Food Chemistry 167:402–408. doi: 10.1016/j.foodchem.2014.07.002.
- Kochetkova, A. A., N. P. Soboleva, L. G. Ipatova, B. A. Shenderov, A. F. Doronin, and E. V. Chizhikova. 2004. Functional soya-based food product. Patent No. RU2236156, filed December 17, 2002, and issued September 20, 2004.
- Kondo, Y., I. Tamura, and Y. Miyagawa. 1994. Fading prevention agent. Patent No. JPH06207172, filed January 11, 1993, and issued July 26, 1994.
- Kopec, R. E., K. M. Riedl, E. H. Harrison, R. W. Curley, Jr, D. P. Hruszkewycz, S. K. Clinton, and S. J. Schwartz. 2010. Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma. Journal of Agricultural and Food Chemistry 58 (6):3290–3296. doi: 10.1021/jf100415z.
- Kopsell, D. A., C. E. Sams, and R. C. Morrow. 2017. Interaction of light quality and fertility on biomass, shoot pigmentation and xanthophyll cycle flux in Chinese kale. Journal of the Science of Food and Agriculture 97 (3):911–917. doi: 10.1002/jsfa.7814.
- Kotíková, Z., M. Šulc, J. Lachman, V. Pivec, M. Orsák, and K. Hamouz. 2016. Carotenoid profile and retention in yellow-, purple- and red-fleshed potatoes after thermal processing . Food Chemistry 197 (Pt A):992–1001. doi: 10.1016/j.foodchem.2015.11.072.
- Kubo, M. T. K., P. E. D. Augusto, and M. Cristianini. 2013. Effect of high pressure homogenization (HPH) on the physical stability of tomato juice. Food Research International 51 (1):170–179. doi: 10.1016/j.foodres.2012.12.004.
- Kubo, M. T. K., D. Maus, A. A. O. Xavier, A. Z. Mercadante, and W. H. Viotto. 2013. Transference of Lutein during cheese making, color stability, and sensory acceptance of Prato cheese. Cienecia e Technologia de Alimentos 33 (Supl 1):81–88.
- Kuhl, J., J. E. Aurich, M. Wulf, A. Hurtienne, F. J. Schweigert, and C. Aurich. 2012. Effects of oral supplementation with β-carotene on concentrations of β-carotene, vitamin A and α-tocopherol in plasma, colostrum and milk of mares and plasma of their foals and on fertility in mares. Journal of Animal Physiology and Animal Nutrition 96 (3):376–384. doi: 10.1111/j.1439-0396.2011.01150.x.
- Kurilich, A. C., and J. A. Juvik. 1999. Quantification of Carotenoid and Tocopherol Antioxidants in Zea mays. Journal of Agricultural and Food Chemistry 47 (5):1948–55. doi: 10.1021/jf981029d.
- Laganà, P., E. Avventuroso, G. Romano, M. E. Gioffré, P. Patanè, S. Parisi, U. Moscato, and S. Delia. 2017. Classification and technological purposes of food additives: The European point of view. In Chemistry and Hygiene of Food Additives, 1–21. Cham, Switzerland: Springer.
- Lakeh, A. A. B., M. R. Ahmadi, S. Safi, T. Ytrestøyl, and B. Bjerkeng. 2010. Growth performance, mortality and carotenoid pigmentation of fry offspring as affected by dietary supplementation of astaxanthin to female rainbow trout (Oncorhynchus mykiss) broodstock. Journal of Applied Ichthyology 26 (1):35–39. doi: 10.1111/j.1439-0426.2009.01349.x.
- Lamberts, L., and J. A. Delcour. 2008. Carotenoids in raw and parboiled brown and milled rice. Journal of Agricultural and Food Chemistry 56 (24):11914–19. doi: 10.1021/jf802613c.
- Lamers, P. P., C. C. van de Laak, P. S. Kaasenbrood, J. Lorier, M. Janssen, R. C. De Vos, R. J. Bino, and R. H. Wijffels. 2010. Carotenoid and fatty acid metabolism in light-stressed Dunaliella salina . Biotechnology and Bioengineering 106 (4):638–648. doi: 10.1002/bit.22725.]
- Lavecchia, R., and A. Zuorro. 2008. Improved lycopene extraction from tomato peels using cell-wall degrading enzymes. European Food Research and Technology 228 (1):153–8. doi: 10.1007/s00217-008-0897-8.
- Lavelli, V., W. Kerr, and P. S. C. Sri Harsha. 2013. Phytochemical stability in dried tomato pulp and peel as affected by moisture properties. Journal of Agricultural and Food Chemistry 61 (3):700–7. doi: 10.1021/jf303987v.
- Lavelli, V., B. Zanoni, and A. Zaniboni. 2007. Effect of water activity on carotenoid degradation in dehydrated carrots. Food Chemistry 104 (4):1705–11. doi: 10.1016/j.foodchem.2007.03.033.
- Leclercq, E., J. R. Dick, J. F. Taylor, J. G. Bell, D. Hunter, and H. Migaud. 2010. Seasonal variations in skin pigmentation and flesh quality of Atlantic salmon (Salmo salar L.): Implications for quality management. Journal of Agricultural and Food Chemistry 58 (11):7036–45. doi: 10.1021/jf100723b.
- Lee, C. G., and J. K. Park. 2010. Fixation of astaxanthin which is estracted from photosynthetic microalgae. Patent No. KR20100030327, filed September 10, 2008, and issued March 18, 2010.
- Lee, J. J. L., L. Chen, J. Shi, A. Trzcinski, and W. Chen. 2014. Metabolomic Profiling of Rhodosporidium toruloides Grown on Glycerol for Carotenoid Production during Different Growth Phases. Journal of Agricultural and Food Chemistry 62 (41):10203–9. doi: 10.1021/jf502987q.
- Leong, S. Y., and I. Oey. 2012. Effects of processing on anthocyanins, carotenoids and vitamin C in summer fruits and vegetables. Food Chemistry 133 (4):1577–87. doi: 10.1016/j.foodchem.2012.02.052.
- Lepage, M., and R. P. A. Sims. 1968. Carotenoids of wheat flour: Their identification and composition. Cereal Chemistry 45:600–4.
- Lerfall, J., E. A. Bendiksen, J. V. Olsen, and M. Østerlie. 2016. A comparative study of organic- versus conventional Atlantic salmon. II. Fillet color, carotenoid- and fatty acid composition as affected by dry salting, cold smoking and storage. Aquaculture 451:369–76. doi: 10.1016/j.aquaculture.2015.10.004.
- Lerfall, J., E. A. Bendiksen, J. V. Olsen, D. Morrice, and M. Østerlie. 2016. A comparative study of organic- versus conventional farmed Atlantic salmon. I. Pigment and lipid content and composition, and carotenoid stability in ice-stored fillets. Aquaculture 451:170–7. doi: 10.1016/j.aquaculture.2015.09.013.
- Leth, T., J. Jakobsen, and N. L. Andersen. 2000. The intake of carotenoids in Denmark. European Journal of Lipid Science and Technology 102 (2):128–32. doi: 10.1002/(SICI)1438-9312(200002)102:2<128::AID-EJLT128>3.0.CO;2-H.
- Leuenberger, B. 2004. Novel stabilized carotenoid compositions. Patent No. EP1418822, filed August 14, 2002, and issued May 19, 2004.
- Leuenberger, B. H., B. Schlegel, and K. M. Voelker. 2004. Process for the manufacture of a powder containing carotenoids. Patent No. US2010136177, filed February 4, 2008, and issued June 3, 2010.
- Li, B., H. Zhao, J. Liu, W. Liu, S. Fan, G. Wu, and R. Zhao. 2015. Application of ultra-high performance supercritical fluid chromatography for the determination of carotenoids in dietary supplements. Journal of Chromatography. A 1425:287–92. doi: 10.1016/j.chroma.2015.11.029.
- Li, Q., and C. Kubota. 2009. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environmental and Experimental Botany 67 (1):59–64. doi: 10.1016/j.envexpbot.2009.06.011.
- Li, Y. 2016. Reinforcing edible vegetable oil rich in natural carotenoid and making method thereof. Patent No. CN106085586, filed June 20, 2016, and issued November 9, 2016.
- Liaaen-Jensen, S., and B. F. Lutnaes. 2008. E/Z isomers and isomerization. In Carotenoids, eds. G. Britton, S. Liaaen-Jensen, and H. Pfander, vol. 4, 15–36. Basel, Boston, Berlin: Birkhäuser.
- Liang, Q., and S. Shao. 2017. Method for extracting carotenoid monomer pigment mainly containing zeaxanthin from wolfberries. Patent No. CN106543769, filed October 31, 2016, and issued March 29, 2017.
- Lim, A. S. L., Z. Burdikova, J. J. Sheehan, and Y. H. Roos. 2016. Carotenoids Stability in High Total Solids Spray Dried Emulsions with gum Arabic Layered Interface and Trehalose-WPI Composites as Wall Materials. Innovative Food Science & Emerging Technologies 34:310–9. doi: 10.1016/j.ifset.2016.03.001.
- Lin, C., and D. Shalitin. 2003. Cryptochrome structure and signal transduction. Annual Review of Plant Biology 54 (1):469–96. doi: 10.1146/annurev.arplant.54.110901.160901.
- Lin, X., and C. Wang. 2014. Method for extracting carotenoid from Melaleuca bracteata, Patent No. CN104003919, filed June 3, 2014, and issued August 27, 2014.
- Lindqvist, H., E. Nadeau, and S. K. Jensen. 2012. Alpha-tocopherol and β-carotene in legume–grass mixtures as influenced by wilting, ensiling and type of silage additive. Grass and Forage Science 67 (1):119–28. doi: 10.1111/j.1365-2494.2011.00827.x.
- Liu, D., J. Li, W. Su, J. Li, X. Hu, H. You, and J. Han. 2005. Method for extracting compound of carotenoid and flavonoid from leaves of purple operilla, Patent No. CN1687029, filed March 28, 2005, and issued October 26, 2005.
- Liu, G. N., Y. H. Zhu, and J. G. Jiang. 2009. The metabolomics of carotenoids in engineered cell factory. Applied Microbiology and Biotechnology 83 (6):989–99. doi: 10.1007/s00253-009-2069-6.
- Liu, L. H., D. Zabaras, L. E. Bennett, P. Aguas, and B. W. Woonton. 2009. Effects of UV-C, red light and sun light on the carotenoid content and physical qualities of tomatoes during post-harvest storage. Food Chemistry 115 (2):495–500. doi: 10.1016/j.foodchem.2008.12.042.
- Liu, Y. Q., C. R. Davis, S. T. Schmaelzle, T. Rocheford, M. E. Cook, and S. A. Tanumihardjo. 2012. β-Cryptoxanthin biofortified maize (Zea mays) increases β-cryptoxanthin concentration and enhances the color of chicken egg yolk. Poultry Science 91 (2):432–8. doi: 10.3382/ps.2011-01719.
- López, A., G.-A. Javier, J. Fenoll, P. Hellín, and P. Flores. 2014. Chemical composition and antioxidant capacity of lettuce: Comparative study of regular-sized (Romaine) and baby-sized (Little Gem and Mini Romaine) types. Journal of Food Composition and Analysis 33 (1):39–48. doi: 10.1016/j.jfca.2013.10.001.
- Lopez, R. S., and E. D. C. L. Cervantes. 2007. Process for the obtention of an oleoresin from ditaxis heterantha zucc; euphorbiaceae seeds. Patent No. MXJL05000040, filed September 30, 2005, and issued March 30, 2007.
- Ma, G., L. Zhang, M. Kato, K. Yamawaki, Y. Kiriiwa, M. Yahata, Y. Ikoma, and H. Matsumoto. 2012. Effect of blue and red LED light irradiation on β-cryptoxanthin accumulation in the flavedo of citrus fruits. Journal of Agricultural and Food Chemistry 60 (1):197–201. doi: 10.1021/jf203364m.
- Ma, G., L. Zhang, M. Kato, K. Yamawaki, Y. Kiriiwa, M. Yahata, Y. Ikoma, and H. Matsumoto. 2015. Effect of the combination of ethylene and red LED light irradiation on carotenoid accumulation and carotenogenic gene expression in the flavedo of citrus fruit. Postharvest Biology and Technology 99:99–104. doi: 10.1016/j.postharvbio.2014.08.002.
- Ma, L., and X.-M. Lin. 2010. Effects of lutein and zeaxanthin on aspects of eye health. Journal of the Science of Food and Agriculture 90 (1):2–12. doi: 10.1002/jsfa.3785.
- Ma, Y., Z. Zhong, J. Zhong, Y. Fang, and J. Wang. 2017. Method for improving content of carotenoid in Lycium barbarum extracting solution and method for preparing Lycium barbarum fermentation drink. Patent No. CN107028065, filed May 31, 2017, and issued August 11, 2017.
- Maiani, G., M. J. Periago Castón, G. Catasta, E. Toti, I. G. Cambrodón, A. Bysted, F. Granado-Lorencio, B. Olmedilla-Alonso, P. Knuthsen, M. Valoti, et al. 2009. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Molecular Nutrition & Food Research 53 (S2):S194–S218.
- Manach, C., J. Hubert, R. Llorach, and A. Scalbert. 2009. The complex links between dietary phytochemicals and human health deciphered by metabolomics. Molecular Nutrition & Food Research 53 (10):1303–15. doi: 10.1002/mnfr.200800516.
- Maoka, T. 2011. Carotenoids in marine animals. Marine Drugs 9 (2):278–93. doi: 10.3390/md9020278.
- Maoka, T. 2016. Structural studies of carotenoids in plants, animals, and food products. In Carotenoids: Nutrition, analysis and technology, eds. A. Kaczor and M. Baranska. Chichester, UK: John Wiley & Sons.
- Mapelli-Brahm, P., F. J. Barba, F. Remize, C. Garcia, A. Fessard, A. Mousavi, A. S. Sant, J. M. Lorenzo, D. Montesano, and A. J. Meléndez-Martínez. 2020. The impact of fermentation processes on the production, retention and bioavailability of carotenoids : An overview. Trends in Food Science & Technology 99:389–401. doi: 10.1016/j.tifs.2020.03.013.
- Mapelli-Brahm, P., C. M. Stinco, M. J. Rodrigo, L. Zacarías, and A. J. Meléndez-Martínez. 2018. Impact of thermal treatments on the bioaccessibility of phytoene and phytofluene in relation to changes in the microstructure and size of orange juice particles. Journal of Functional Foods 46:38–47. doi: 10.1016/j.jff.2018.04.044.
- Mares, D. J., and A. W. Campbell. 2001. Mapping components of flour colour in Australian wheat. Australian Journal of Agricultural Research 52 (12):1297–309. doi: 10.1071/AR01048.
- Mariutti, L. R., and A. Z. Mercadante. 2018. Carotenoid esters analysis and occurrence: What do we know so far? Archives of Biochemistry and Biophysics 648:36–43. doi: 10.1016/j.abb.2018.04.005.
- Marles, M. A., T. D. Warkentin, and K. E. Bett. 2013. Genotypic abundance of carotenoids and polyphenolics in the hull of field pea (Pisum sativum L.). Journal of the Science of Food and Agriculture 93 (3):463–70. doi: 10.1002/jsfa.5782.
- Martin, B., V. Fedele, A. Ferlay, P. Grolier, E. Rock, D. Gruffat, and Y. Chilliard. 2004. Effects of grass-based diets on the content of micronutrients and fatty acids in bovine and caprine dairy products. In Land use systems in grassland dominated regions, eds. A. Luscher, B. Jeangros, W. Kessler, O. Huguenin, M. Lobsiger, N. Millar, and D. Suter, vol. 9, 876–86. Zürich, Switzerland: vdf.
- Martin-Moreno, J. M., and L. Gorgojo. 2007. Valoración de la ingesta dietética a nivel poblacional mediante cuestionarios individuales: Sombras y luces metodológicas. Revista Española de Salud Pública 81 (5):507–18. doi: 10.1590/S1135-57272007000500007.
- Martínez-Hernández, G. B., M. Boluda-Aguilar, A. Taboada-Rodríguez, S. Soto-Jover, F. Marín-Iniesta, and A. López-Gómez. 2016. Processing, Packaging, and Storage of Tomato Products: Influence on the Lycopene Content. Food Engineering Reviews 8 (1):52–75. doi: 10.1007/s12393-015-9113-3.
- Masone, D., and C. Chanforan. 2015. Study on the interaction of artificial and natural food colorants with human serum albumin: A computational point of view. Computational Biology and Chemistry 56:152–8. doi: 10.1016/j.compbiolchem.2015.04.006.
- Matsumoto, M., H. Hara, M. Hosokawa, K. Miyashita, and M. Nishimukai. 2008. Composition for controlling absorption of neutral fat. Patent No. JP2008231057, filed March 22, 2007, and issued October 2, 2018.
- Mditshwa, A., L. S. Magwaza, S. Z. Tesfay, and N. C. Mbili. 2017. Effect of ultraviolet irradiation on postharvest quality and composition of tomatoes: A review. Journal of Food Science and Technology 54 (10):3025–35. doi: 10.1007/s13197-017-2802-6.
- Meléndez-Martínez, A. J. 2019. An overview of carotenoids, apocarotenoids and vitamin A in agro-food, nutrition, health and disease. Molecular Nutrition and Food Research 180:1045.
- Meléndez-Martínez, A. J. J., G. Britton, I. M. I. M. Vicario, and F. J. F. J. Heredia. 2007. Relationship between the colour and the chemical structure of carotenoid pigments. Food Chemistry 101 (3):1145–50. doi: 10.1016/j.foodchem.2006.03.015.
- Meléndez-Martínez, A. J., P. Mapelli-Brahm, and C. M. Stinco. 2018. The colourless carotenoids phytoene and phytofluene: From dietary sources to their usefulness for the functional foods and nutricosmetics industries. Journal of Food Composition and Analysis 67:91–103. doi: 10.1016/j.jfca.2018.01.002.
- Meléndez-Martínez, A. J., P. Mapelli-Brahm, A. Benítez-González, and C. M. Stinco. 2015. A comprehensive review on the colorless carotenoids phytoene and phytofluene. Archives of Biochemistry and Biophysics 572:188–200. doi: 10.1016/j.abb.2015.01.003.
- Meléndez-Martínez, A., C. M. Stinco, C. Liu, and X.-D. Wang. 2013. A simple HPLC method for the comprehensive analysis of cis/trans (Z/E) geometrical isomers of carotenoids for nutritional studies. Food Chemistry 138 (2–3):1341–50. doi: 10.1016/j.foodchem.2012.10.067.
- Mellado-Ortega, E., and D. Hornero-Méndez. 2012. Isolation and identification of lutein esters, including their regioisomers, in tritordeum (×Tritordeum Ascherson et Graebner) grains: Evidence for a preferential xanthophyll acyltransferase activity. Food Chemistry 135 (3):1344–52. doi: 10.1016/j.foodchem.2012.05.046.
- Mellado-Ortega, E., and D. Hornero-Méndez. 2015a. Carotenoid profiling of Hordeum chilense grains: The parental proof for the origin of the high carotenoid content and esterification pattern of tritordeum. Journal of Cereal Science 62:15–21. doi: 10.1016/j.jcs.2014.12.005.
- Mellado-Ortega, E., and D. Hornero-Méndez. 2015b. Carotenoids in cereals: An ancient resource with present and future applications. Phytochemistry Reviews 14 (6):873–90. doi: 10.1007/s11101-015-9423-3.
- Mellado-Ortega, E., and D. Hornero-Méndez. 2016. Carotenoid evolution during short-storage period of durum wheat (Triticum turgidum conv. durum) and tritordeum (×Tritordeum Ascherson et Graebner) whole-grain flours. Food Chemistry 192:714–23. doi: 10.1016/j.foodchem.2015.07.057.
- Mellado-Ortega, E., and D. Hornero-Méndez. 2017. Effect of long-term storage on the free and esterified carotenoids in durum wheat (Triticum turgidum conv. durum) and tritordeum (×Tritordeum Ascherson et Graebner) grains. Food Research International (Ottawa, Ont.) 99 (Pt 2):877–90. et Graebner) grains. (2), doi: 10.1016/j.foodres.2016.05.012.
- Meng, D., J. Wang, X. Li, Z. Tian, Z. Chen, L. Lan, Y. Wu, and S. Feng. 2014. Carotenoid hollow fiber membrane liquid-phase micro-extraction method. Patent No. CN104034572, filed June 24, 2014, and issued September 10, 2014.
- Mercadante, A. Z., D. B. Rodrigues, F. C. Petry, and L. R. B. Mariutti. 2017. Carotenoid esters in foods - A review and practical directions on analysis and occurrence. Food Research International (Ottawa, Ont.) 99 (Pt 2):830–50. doi: 10.1016/j.foodres.2016.12.018.
- Merzlyak, M. N., T. B. Melø, and K. R. Naqvi. 2008. Effect of anthocyanins, carotenoids, and flavonols on chlorophyll fluorescence excitation spectra in apple fruit: Signature analysis, assessment, modelling, and relevance to photoprotection. Journal of Experimental Botany 59 (2):349–59. doi: 10.1093/jxb/erm316.
- Mi, J., K. P. Jia, A. Balakrishna, and S. Al-Babili. 2020. A method for extraction and LC-MS-based identification of carotenoid-derived dialdehydes in plants. In Plant and food carotenoids, vol. 2083, 177–88. New York, NY: Humana Press.
- Mínguez-Mosquera, M. I., D. Hornero-Méndez, and A. Pérez-Gálvez. 2008. Analysis of carotenoids and provitamin A in functional foods. In Methods of analysis for functional foods and nutraceuticals, ed. W. J Hurst, 2nd ed. Boca Raton, FL: CRC Press LLC.
- Misawa, N., H. Harada, and T. Maoka. 2014. Method for extracting carotenoid from plant. Patent No. JP2014050374, filed September 10, 2012, and issued Mrch 20, 2014.
- Monge-Rojas, R., and H. Campos. 2011. Tocopherol and carotenoid content of foods commonly consumed in Costa Rica. Journal of Food Composition and Analysis 24 (2):202–16. doi: 10.1016/j.jfca.2010.09.015.
- Mora, O., J. L. Romano, E. Gonzalez, F. J. Ruiz, R. Gomez, and A. Shimada. 2001. Presence of fed beta-carotene in digesta, excreta, blood, and hepatic and adipose tissues of Holstein steers. Canadian Journal of Animal Science 81 (1):133–9. doi: 10.4141/A00-004.
- Moros, E. E., D. Darnoko, M. Cheryan, E. G. Perkins, and J. Jerrell. 2002. Analysis of xanthophylls in corn by HPLC. Journal of Agricultural and Food Chemistry 50 (21):5787–90. doi: 10.1021/jf020109l.
- Muhin, V. A., V. J. Novikov, L. A. Sapovalova, and O. A. Seveleva. 2005. Method for production of fat-soluble carotenoid complex from hydrobios and waste from reprocessing thereof. Patent No. RU2004100513, filed January 5, 2004, and issued June 10, 2005.
- Müller, C. E., J. Möller, S. K. Jensen, and P. Udén. 2007. Tocopherol and carotenoid levels in baled silage and haylage in relation to horse requirements. Animal Feed Science and Technology 137 (1–2):182–97. doi: 10.1016/j.anifeedsci.2006.10.007.
- Müller-Maatsch, J., J. Sprenger, J. Hempel, F. Kreiser, R. Carle, and R. M. Schweiggert. 2016. Carotenoids from gac fruit aril (Momordica cochinchinensis [Lour.] Spreng.) are more bioaccessible than those from carrot root and tomato fruit. Food Research International 99:2, 928–935.
- Mun, O. S., J. N. Moon, M. K. Roh, M. H. Jeon, I. S. Choi, and J. S. Choi. 2012. Method for obtaining purified lycopene and water-soluble lycopene from tomato. Patent No. KR101112053, filed June 20, 2011, and issued February 13, 2012.
- Murillo, E., D. Giuffrida, D. Menchaca, P. Dugo, G. Torre, A. J. Meléndez-Martinez, and L. Mondello. 2013. Native carotenoids composition of some tropical fruits. Food Chemistry 140 (4):825–36. doi: 10.1016/j.foodchem.2012.11.014.
- Murillo, E., A. J. Meléndez-Martínez, and F. Portugal. 2010. Screening of vegetables and fruits from Panama for rich sources of lutein and zeaxanthin. Food Chemistry 122 (1):167–72. doi: 10.1016/j.foodchem.2010.02.034.
- Murkovic, M., K. Gams, S. Draxl, and W. Pfannhauser. 2000. Development of an Austrian carotenoid database. Journal of Food Composition and Analysis 13 (4):435–40. doi: 10.1006/jfca.2000.0909.
- Musaeus, N., and C. N. Jensen. 2007. Method for producing an aqueous suspension and a powdered preparation of one or more carotinoids. Patent No. DE102005030952, filed June 30, 2005, and issued January 18, 2007.
- Mustafa, A., and C. Turner. 2011. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Analytica Chimica Acta 703 (1):8–18. doi: 10.1016/j.aca.2011.07.018.
- Mustafa, A., L. Mijangos, and C. Turner. 2012. Pressurized hot ethanol extraction of carotenoids from carrot by-products. Molecules (Basel, Switzerland) 17 (2):1809–18. doi: 10.3390/molecules17021809.
- Náthia-Neves, G., and M. A. M. Meireles. 2018. Genipap: A New Perspective on Natural Colorants for the Food Industry. Food and Public Health 8 (1):21–33.
- Neumann, S., and S. Böcker. 2010. Computational mass spectrometry for metabolomics: identification of metabolites and small molecules. Analytical and Bioanalytical Chemistry 398 (7–8):2779–88. doi: 10.1007/s00216-010-4142-5.
- Nielen, M. W. F., M. C. Van Engelen, R. Zuiderent, and R. Ramaker. 2007. Screening and confirmation criteria for hormone residue analysis using liquid chromatography accurate mass time-of-flight, Fourier transform ion cyclotron resonance and orbitrap mass spectrometry techniques. Analytica Chimica Acta 586 (1–2):122–9. doi: 10.1016/j.aca.2006.08.055.
- Nimalaratne, C., D. Lopes-Lutz, A. Schieber, and J. Wu. 2012. Effect of domestic cooking methods on egg yolk xanthophylls. Journal of Agricultural and Food Chemistry 60 (51):12547–52. doi: 10.1021/jf303828n.
- Nozière, P., B. Graulet, A. Lucas, B. Martin, P. Grolier, and M. Doreau. 2006. Carotenoids for ruminants: From forages to dairy products. Animal Feed Science and Technology 131 (3–4):418–50. doi: 10.1016/j.anifeedsci.2006.06.018.
- Nozière, P., P. Grolier, D. Durand, A. Ferlay, P. Pradel, and B. Martin. 2006. Variations in carotenoids, fat-soluble micronutrients, and color in cows’ plasma and milk following changes in forage and feeding level. Journal of Dairy Science 89 (7):2634–48. doi: 10.3168/jds.S0022-0302(06)72340-2.
- O’Callaghan, T. F., D. T. Mannion, D. Hennessy, S. McAuliffe, M. G. O'Sullivan, N. Leeuwendaal, T. P. Beresford, P. Dillon, K. N. Kilcawley, J. J. Sheehan, et al. 2017. Effect of pasture versus indoor feeding systems on quality characteristics, nutritional composition, and sensory and volatile properties of full-fat Cheddar cheese . Journal of Dairy Science 100 (8):6053–73. doi: 10.3168/jds.2016-12508.
- O’Neill, M. E., Y. Carroll, B. Corridan, B. Olmedilla, F. Granado, I. Blanco, H. V. d Berg, I. Hininger, A.-M. Rousell, M. Chopra, et al. 2001. A European carotenoid database to assess carotenoid intakes and its use in a five-country comparative study. British Journal of Nutrition 85 (4):499–507. doi: 10.1079/BJN2000284.
- Oliveira, A., E. M. C. Alexandre, M. Coelho, R. M. Barros, D. P. F. Almeida, and M. Pintado. 2016. Peach polyphenol and carotenoid content as affected by frozen storage and pasteurization. LWT - Food Science and Technology 66:361–8. doi: 10.1016/j.lwt.2015.10.037.
- Olmedilla-Alonso, B., B. Beltrán-de-Miguel, R. Estévez-Santiago, and C. Cuadrado-Vives. 2014. Markers of lutein and zeaxanthin status in two age groups of men and women: Dietary intake, serum concentrations, lipid profile and macular pigment optical density. Nutrition Journal 13 (1):52. doi: 10.1186/1475-2891-13-52.
- Oplatowska-Stachowiak, M., and C. T. Elliott. 2017. Food colors: Existing and emerging food safety concerns. Critical Reviews in Food Science and Nutrition 57 (3):524–48. doi: 10.1080/10408398.2014.889652.
- Ordóñez-Santos, L. E., J. Martínez-Girón, and M. E. Arias-Jaramillo. 2017. Effect of ultrasound treatment on visual color, vitamin C, total phenols, and carotenoids content in Cape gooseberry juice. Food Chemistry 233:96–100. doi: 10.1016/j.foodchem.2017.04.114.
- Ouzounis, T., B. Razi Parjikolaei, X. Fretté, E. Rosenqvist, and C.-O. Ottosen. 2015. Predawn and high intensity application of supplemental blue light decreases the quantum yield of PSII and enhances the amount of phenolic acids, flavonoids, and pigments in Lactuca sativa. Frontiers in Plant Science 6:19–4. doi: 10.3389/fpls.2015.00019.
- Ouzounis, T., E. Rosenqvist, and C.-O. Ottosen. 2015. Spectral effects of artificial light on plant physiology and secondary metabolism. HortScience 50 (8):1128–35. doi: 10.21273/HORTSCI.50.8.1128.
- Ozegowski, J.-H., P.-J. Mueller, A. Christner, and A. Siering. 2006. Method for extracting carotenoid from damp biomasses, comprises milling and moisture evaporating the biomass; mixing the obtained product with extracting agents of an oil or a fat; and thermally treating and extracting the carotenoid. Patent No. DE102005007885, filed February 16, 2005, and issued August 24, 2006.
- Panfili, G., A. Fratianni, and M. Irano. 2004. Improved normal-phase high-performance liquid chromatography procedure for the determination of carotenoids in cereals. Journal of Agricultural and Food Chemistry 52 (21):6373–7. doi: 10.1021/jf0402025.
- Panjai, L., G. Noga, A. Fiebig, and M. Hunsche. 2017. Effects of continuous red light and short daily UV exposure during postharvest on carotenoid concentration and antioxidant capacity in stored tomatoes. Scientia Horticulturae 226:97–103. doi: 10.1016/j.scienta.2017.08.035.
- Patterson, R. E., and P. Pietinen. 2006. Evaluación del estado nutricional en individuos y poblacions. In Nutrición y Salud Pública, eds. M. L. Gibney, B. M. Margetts, J. M. Kearney, and L. Arab. Oxford, UK: Blackwell Science Ltd, and Ed. S. A. Acribia (Zaragoza, España), para la versión española.
- Pereira, D. M., P. Valentão, and P. B. Andrade. 2014. Marine natural pigments: Chemistry, distribution and analysis. Dyes and Pigments 111:124–34. doi: 10.1016/j.dyepig.2014.06.011.
- Pérez-Conesa, D., J. García-Alonso, V. García-Valverde, M.-D. Iniesta, K. Jacob, L. M. Sánchez-Siles, G. Ros, and M. J. Periago. 2009. Changes in bioactive compounds and antioxidant activity during homogenization and thermal processing of tomato puree. Innovative Food Science & Emerging Technologies 10 (2):179–88. doi: 10.1016/j.ifset.2008.12.001.
- Pérez-Fernández, V., S. Ventura, P. Tomai, R. Curini, and A. Gentili. 2017. Determination of target fat-soluble micronutrients in rainbow trout’s muscle and liver tissues by liquid chromatography with diode array-tandem mass spectrometry detection. Electrophoresis 38 (6):886–96. doi: 10.1002/elps.201600427.
- Perry, A., H. Rasmussen, and E. J. Johnson. 2009. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. Journal of Food Composition and Analysis 22 (1):9–15. doi: 10.1016/j.jfca.2008.07.006.
- Pertuzatti, P. B., M. Sganzerla, A. C. Jacques, M. T. Barcia, and R. C. Zambiazi. 2015. Carotenoids, tocopherols and ascorbic acid content in yellow passion fruit (Passiflora edulis) grown under different cultivation systems. LWT - Food Science and Technology 64 (1):259–63. doi: 10.1016/j.lwt.2015.05.031.
- Pezdirc, K., M. J. Hutchesson, R. L. Williams, M. E. Rollo, T. L. Burrows, L. G. Wood, C. Oldmeadow, and C. E. Collins. 2016. Consuming high-carotenoid fruit and vegetables influences skin yellowness and plasma carotenoids in young women: A single-blind randomized crossover trial. Journal of the Academy of Nutrition and Dietetics 116 (8):1257–65. doi: 10.1016/j.jand.2016.03.012.
- Pham, M. A., H. G. Byun, K.-D. Kim, and S.-M. Lee. 2014. Effects of dietary carotenoid source and level on growth, skin pigmentation, antioxidant activity and chemical composition of juvenile olive flounder Paralichthys olivaceus. Aquaculture 431:65–72. doi: 10.1016/j.aquaculture.2014.04.019.
- Pickworth, C. L., S. C. Loerch, R. E. Kopec, S. J. Schwartz, and F. L. Fluharty. 2012. Concentration of pro-vitamin A carotenoids in common beef cattle feedstuffs. Journal of Animal Science 90 (5):1553–61. doi: 10.2527/jas.2011-4217.
- Pinto De Abreu, F. A., M. Dornier, D. Pallet, M. Reynes, F. Vaillant, and F. C. Torres Furlani. 2019. Concentration and purification of extract obtained from cashew pseudofruit wastes and products with a high carotenoid content. Patent No. AU2016253666, filed November 21, 2019, and issued December 12, 2019.
- Poiroux-Gonord, F., L. P. R. Bidel, A. L. Fanciullino, H. Gautier, F. Lauri-Lopez, and L. Urban. 2010. Health benefits of vitamins and secondary metabolites of fruits and vegetables and prospects to increase their concentrations by agronomic approaches. Journal of Agricultural and Food Chemistry 58 (23):12065–82. doi: 10.1021/jf1037745.
- Pokhrel, P. R., D. Bermúdez-Aguirre, H. E. Martínez-Flores, M. G. Garnica-Romo, S. Sablani, J. Tang, and G. V. Barbosa-Cánovas. 2017. Combined Effect of Ultrasound and Mild Temperatures on the Inactivation of E. coli in Fresh Carrot Juice and Changes on its Physicochemical Characteristics. Journal of Food Science 82 (10):2343–50. doi: 10.1111/1750-3841.13787.
- Pop, R. M., Y. Weesepoel, C. Socaciu, A. Pintea, J.-P. Vincken, and H. Gruppen. 2014. Carotenoid composition of berries and leaves from six Romanian Sea Buckthorn (Hippophae rhamnoides L.) varieties. Food Chemistry 147:1–9. doi: 10.1016/j.foodchem.2013.09.083.
- Postoienko, V. O., O. M. Postoienko, A. L. Boiko, and M. Y. Kucherenko. 2004. Method for producing oil carotenoid concentrate from green mass of plant material. Patent No. UA73556, filed October 10, 2002, and issued April 15, 2004.
- Prache, S., N. Kondjoyan, O. Delfosse, B. Chauveau-Duriot, D. Andueza, and A. Cornu. 2009. Discrimination of pasture-fed lambs from lambs fed dehydrated alfalfa indoors using different compounds measured in the fat, meat and plasma. Animal 3 (4):598–605. doi: 10.1017/S1751731108003881.
- Prache, S., A. Priolo, and P. Grolier. 2003a. Effect of concentrate finishing on the carotenoid content of perirenal fat in grazing sheep: Its significance for discriminating grass-fed, concentrate-fed and concentrate-finished grazing lambs. Animal Science 77 (2):225–33. doi: 10.1017/S1357729800058963.
- Prache, S., A. Priolo, and P. Grolier. 2003b. Persistence of carotenoid pigments in the blood of concentratefinished grazing sheep: Its significance for the traceability of grass-feeding. Journal of Animal Science 81 (2):360–7. doi: 10.2527/2003.812360x.
- Qiaosheng, G., S. Haijin, and L. Li. 2011. Extraction method of wild chrysanthemum carotenoid pigment and perpetration method thereof. Patent No. CN101967302, filed October 22, 2010, and issued February 9, 2011.
- Qin, G., Z. Wang, H. Pang, and L. Gu. 2014. Method for preparing corn bran oil through combination of tera-hertz radiation and sub-critical extraction. Patent No. CN103756780, filed January 8, 2014, and issued April 30, 2014.
- Quail, P. H., M. T. Boylan, B. M. Parks, T. W. Short, Y. Xu, and D. Wagner. 1995. Phytochromes: Photosensory perception and signal transduction. Science (New York, N.Y.) 268 (5211):675–80. doi: 10.1126/science.7732376.
- Quan, C., and C. Turner. 2009. Extraction of astaxanthin from shrimp waste using pressurized hot ethanol. Chromatographia 70 (1–2):247–51. doi: 10.1365/s10337-009-1113-0.
- Quigley, L., D. J. O’Sullivan, D. Daly, O. O’Sullivan, Z. Burdikova, R. Vana, T. P. Beresford, R. P. Ross, G. F. Fitzgerald, P. L. H. McSweeney, et al. 2016. Thermus and the pink discoloration defect in cheese. MSystems 1 (3):e00023–16. doi: 10.1128/mSystems.00023-16.
- Ram, S., M. Mitra, F. Shah, S. R. Tirkey, S. Mishra, S. Rani, and S. Mishra. 2020. Bacteria as an alternate biofactory for carotenoid production: A review of its applications, opportunities and challenges. Journal of Functional Foods 67 (103867):103867. doi: 10.1016/j.jff.2020.103867.
- Rearte, T. A., F. L. Figueroa, C. Gómez-Serrano, C. G. Vélez, S. Marsili, A. d F. Iorio, C. V. González-López, M. C. Cerón-García, R. T. Abdala-Díaz, and F. G. Acién-Fernández. 2020. Optimization of the production of lipids and carotenoids in the microalga Golenkinia aff. brevispicula. Algal Research 51:102004. doi: 10.1016/j.algal.2020.102004.
- Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. http://data.europa.eu/eli/reg/2008/1333/oj.
- Reif, C., E. Arrigoni, H. Schärer, L. Nyström, and R. F. Hurrell. 2013. Carotenoid database of commonly eaten Swiss vegetables and their estimated contribution to carotenoid intake. Journal of Food Composition and Analysis 29 (1):64–72. doi: 10.1016/j.jfca.2012.10.005.
- Reyes, M. F. A., M. G. Nuñez, and L. J. M. Del Valle. 2015. Method of producing carotenoid compounds from microalgal substrate including compression of the microalgal powder and extraction by supercritical CO2. Patent No. WO2015123789, filed December 24, 2014, and issued August 27, 2015.
- Ribeiro, B. D., D. W. Barreto, and M. A. Z. Coelho. 2011. Technological Aspects of β-Carotene Production. Food and Bioprocess Technology 4 (5):693–701. doi: 10.1007/s11947-011-0545-3.
- Riedl, K. M., K. Choksi, F. J. Wyzgoski, J. C. Scheerens, S. J. Schwartz, and R. N. Reese. 2013. Variation in lycopene and lycopenoates, antioxidant capacity, and fruit quality of buffaloberry (Shepherdia argentea [pursh]nutt.). Journal of Food Science 78 (11):C1673–C1679. doi: 10.1111/1750-3841.12265.
- Rivera, S. M., and R. Canela-Garayoa. 2012. Analytical tools for the analysis of carotenoids in diverse materials. Journal of Chromatography. A 1224:1–10. doi: 10.1016/j.chroma.2011.12.025.
- Robertson, K. D., J. L. Kalbfleisch, W. Pan, and R. A. Charbeneau. 2005. Effect of corn distiller’s dried grains with solubles at various levels on performance of laying hens and egg yolk color. International Journal of Poultry Science 4 (2):44–51. doi: 10.3923/ijps.2005.44.51.
- Roche, H. L. 1965. The manufacture and use of a carotenoid. Patent No. GB1003546, filed Novemer 5, 1963, and issued September 8, 1965.
- Rodríguez-Amaya, D. B. 1997. Carotenoids and food preparation: the retention of provitamin A carotenoids in prepared, processed and stored foods. Arlington, VA: John Snow Incorporated/OMNI Project.
- Rodríguez-Amaya, D. B. 2001. A guide to carotenoid analysis in foods, vol. 71. Washington, DC: ILSI press.
- Rodríguez-Amaya, D. B. 2010. Quantitative analysis, in vitro assessment of bioavailability and antioxidant activity of food carotenoids-A review. Journal of Food Composition and Analysis 23:726–40.
- Rodríguez-Amaya, D. B. 2016a. Composition and influencing factors. In Food carotenoids: Chemistry, biology and technology, 1st ed., 96–109. Hoboken, NJ: John Wiley & Sons.
- Rodríguez-Amaya, D. B. 2016b. Food carotenoids, chemistry, biology and technology. Chichester, UK: IFT Press, Wiley Blackwell.
- Rodríguez-Amaya, D. B., and M. Kimura. 2004. HarvestPlus handbook for carotenoid analysis. Washington, DC: HarvestPlus.
- Rodríguez-Amaya, D. B., M. Kimura, and J. Amaya-Farfan. 2008. Fontes brasileiras de carotenoides: Tabela brasileira de composição de carotenoides em alimentos Brasília: Ministério do Meio Ambiente.
- Rodríguez-Amaya, D. B., M. Kimura, H. T. Godoy, and J. Amaya-Farfan. 2008. Updated Brazilian database on food carotenoids: Factors affecting carotenoid composition. Journal of Food Composition and Analysis 21 (6):445–63. doi: 10.1016/j.jfca.2008.04.001.
- Rodríguez-Concepcion, M., J. Avalos, M. L. Bonet, A. Boronat, L. Gomez-Gomez, D. Hornero-Mendez, M. C. Limon, A. J. Meléndez-Martínez, B. Olmedilla-Alonso, A. Palou, et al. 2018. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Progress in Lipid Research 70:62–93. doi: 10.1016/j.plipres.2018.04.004.
- Rodríguez-Suárez, C., M. J. Giménez, and S. G. Atienza. 2010. Progress and perspectives for carotenoid accumulation in selected Triticeae species. Crop and Pasture Science 61 (9):743–51. doi: 10.1071/CP10025.
- Röhrle, F., A. Moloney, M. Osorio, G. Luciano, A. Priolo, P. Caplan, and F. J. Monahan. 2011. Carotenoid, colour and reflectance measurements in bovine adipose tissue to discriminate between beef from different feeding systems. Meat Science 88 (3):347–53. doi: 10.1016/j.meatsci.2011.01.005.
- Rojas-Garbanzo, C., M. Gleichenhagen, A. Heller, P. Esquivel, N. Schulze-Kaysers, and A. Schieber. 2017. Carotenoid profile, antioxidant capacity, and chromoplasts of pink guava (Psidium guajava L. cv. ‘Criolla’) during fruit ripening. Journal of Agricultural and Food Chemistry 65 (18):3737–47. doi: 10.1021/acs.jafc.6b04560.
- Roncarati, A., F. Sirri, A. Felici, L. Stocchi, P. Melotti, and A. Meluzzi. 2011. Effects of dietary supplementation with krill meal on pigmentation and quality of flesh of rainbow trout (Oncorhynchus mykiss). Italian Journal of Animal Science 10 (2):e27–145. doi: 10.4081/ijas.2011.e27.
- Runge, F., E. Lueddecke, and A.-M. Pfeiffer. 2002. Process to produce dry powder of a carotenoid or several carotenoids. Patent No. DE10104494, filed January 31, 2001, and issued August 1, 2002.
- Sabio, E., M. Lozano, V. Montero de Espinosa, R. L. Mendes, A. P. Pereira, A. F. Palavra, and J. A. Coelho. 2003. Lycopene and β-carotene extraction from tomato processing waste using supercritical CO2. Industrial & Engineering Chemistry Research 42 (25):6641–6.
- Sachindra, N. M., and N. S. Mahendrakar. 2005. Process optimization for extraction of carotenoids from shrimp waste with vegetable oils. Bioresource Technology 96 (10):1195–200. doi: 10.1016/j.biortech.2004.09.018.
- Sachindra, N. M., N. Bhaskar, and N. S. Mahendrakar. 2006. Recovery of carotenoids from shrimp waste in organic solvents. Waste Management 26 (10):1092–8. doi: 10.1016/j.wasman.2005.07.002.
- Saini, R. K., and Y. S. Keum. 2018. Carotenoid extraction methods: A review of recent developments. Food Chemistry 240:90–103. doi: 10.1016/j.foodchem.2017.07.099.
- Samuolienė, G., A. Brazaitytė, R. Sirtautas, A. Viršilė, J. Sakalauskaitė, S. Sakalauskienė, and P. Duchovskis. 2013. LED illumination affects bioactive compounds in romaine baby leaf lettuce. Journal of the Science of Food and Agriculture 93 (13):3286–91. doi: 10.1002/jsfa.6173.
- Sander, L. C., K. E. Sharpless, N. E. Craft, and S. A. Wise. 1994. Development of engineered stationary phases for the separation of carotenoid isomers. Analytical Chemistry 66 (10):1667–74. doi: 10.1021/ac00082a012.
- Sangwan, N. S., P. Tiwari, S. K. Mishra, R. K. Yadav, S. M. Tripathi, A. K. Kuswaha, and R. S. Sangwan. 2015. Plant metabolomics: An overview of technology platforms for applications in metabolism. In PlantOmics: The omics of plant science, eds. D. Barh, M. Khan, and E. Davies. New Delhi, India: Springer.
- Santhirasegaram, V., Z. Razali, and C. Somasundram. 2015. Effects of sonication and ultraviolet-C treatment as a hurdle concept on quality attributes of Chokanan mango (Mangifera indica L.) juice. Food Science and Technology International = Ciencia y Tecnologia de Los Alimentos Internacional 21 (3):232–41. doi: 10.1177/1082013214528188.
- Santos-Bocanegra, E., X. Ospina-Osorio, and E. O. Oviedo-Rondón. 2004. Evaluation of xanthophylls extracted from Tagetes erectus (Marigold flower) and Capsicum Sp. (Red pepper paprika) as a pigment for egg-yolks compare with Synthetic pigments. International Journal of Poultry Science 3 (11):685–9.
- Sarkar, S., M. S. Manna, T. K. Bhowmick, and K. Gayen. 2020. Extraction of chlorophylls and carotenoids from dry and wet biomass of isolated Chlorella Thermophila: Optimization of process parameters and modelling by artificial neural network. Process Biochemistry 96:58–72. doi: 10.1016/j.procbio.2020.05.025.
- Sawada, Y., M. Sato, M. Okamoto, J. Masuda, S. Yamaki, M. Tamari, Y. Tanokashira, S. Kishimoto, A. Ohmiya, T. Abe, et al. 2019. Metabolome-based discrimination of chrysanthemum cultivars for the efficient generation of flower color variations in mutation breeding. Metabolomics 15 (9):118. doi: 10.1007/s11306-019-1573-7.
- Sazykin, I. S., M. A. Sazykina, and V. A. E. Chistjakov. 2013. Method to produce deinoxanthine-carotenoid of microorganism Deinococcus radiodurans. Patent No. RU2475541, filed September 26, 2011, and issued Februarz 20, 2013.
- Schaffner, D. 2006. Process for the manufacture of powderous preparations of fat-soluble substances. Patent No. US2006115534, filed July 6, 2005, and issued June 1, 2006.
- Schaub, P., F. Wüst, J. Koschmieder, Q. Yu, P. Virk, J. Tohme, and P. Beyer. 2017. Nonenzymatic β-Carotene Degradation in Provitamin A-Biofortified Crop Plants. Journal of Agricultural and Food Chemistry 65 (31):6588–98. doi: 10.1021/acs.jafc.7b01693.
- Schiedt, K. 1998. Absorption andmetabolismof carotenoids in birds, fish and crustacean. In Carotenoids, eds. G. Britton, S. Liaaen-Jensen, and H. Pfander, 285–358. Basel, Switzerland: Birkhauser.
- Schlatterer, J., and D. E. Breithaupt. 2006. Xanthophylls in commercial egg yolks: Quantification and identification by HPLC and LC-(APCI) MS using a C30 phase. Journal of Agricultural and Food Chemistry 54 (6):2267–73. doi: 10.1021/jf053204d.
- Schlegel, B., and G. Badolato Boenisch. 2012. Beadlets comprising carotenoids. Patent No. WO2012143492, filed April 20, 2012, and issued October 26, 2012.
- Schüler, L. M., K. N. Gangadhar, P. Duarte, C. Placines, A. M. Molina-Márquez, R. Léon-Bañares, V. S. Sousa, J. Varela, and L. Barreira. 2020. Improvement of carotenoid extraction from a recently isolated, robust microalga, Tetraselmis sp. CTP4 (chlorophyta). Bioprocess and Biosystems Engineering 43 (5):785–96. doi: 10.1007/s00449-019-02273-9.
- Schweigert, F. J. 1998. Metabolism of carotenoids in mammals. In Carotenoids, eds. G Britton, S Liaaen-Jensen, and H. PFander, vol. 3, 249–84. Basel, Berlin, Germany: Birkhäuser, Verlag.
- Schweigert, F. J., and C. Gottwald. 1999. Effect of parturition on levels of vitamins A and E and of beta-carotene in plasma and milk of mares. Equine Veterinary Journal 31 (4):319–23. doi: 10.1111/j.2042-3306.1999.tb03824.x.
- Schweiggert, R. M., and R. Carle. 2017. Carotenoid deposition in plant and animal foods and its impact on bioavailability. Critical Reviews in Food Science and Nutrition 57 (9):1807–30. doi: 10.1080/10408398.2015.1012756.
- Schweiggert, R. M., C. B. Steingass, E. Mora, P. Esquivel, and R. Carle. 2011. Carotenogenesis and physico-chemical characteristics during maturation of red fleshed papaya fruit (Carica papaya L.). Food Research International 44 (5):1373–80. doi: 10.1016/j.foodres.2011.01.029.
- Scialpi, L. J. 1987. Process for preparing fat-soluble vitamin active beadlets. Patent No. US4670247, filed March 24, 1986, and issued June 2, 1987.
- Scott, C. E., and A. L. Eldridge. 2005. Comparison of carotenoid content in fresh, frozen and canned corn. Journal of Food Composition and Analysis 18 (6):551–9. doi: 10.1016/j.jfca.2004.04.001.
- Scott, K. J., D. I. Thurnham, D. J. Hart, S. A. Bingham, and K. Day. 1996. The correlation between the intake of lutein, lycopene and beta-carotene from vegetables and fruits, and blood plasma concentrations in a group of women aged 50-65 years in the UK. The British Journal of Nutrition 75 (3):409–18. doi: 10.1079/bjn19960143.
- Shao, S. 2016. Environment-friendly carotenoid production method. Patent No. CN105648019, filed April 5, 2016, and issued June 8, 2016.
- Shardell, M. D., D. E. Alley, G. E. Hicks, S. S. El-Kamary, R. R. Miller, R. D. Semba, and L. Ferrucci. 2011. Low-serum carotenoid concentrations and carotenoid interactions predict mortality in US adults: The Third National Health and Nutrition Examination Survey. Nutrition Research 31 (3):178–89. doi: 10.1016/j.nutres.2011.03.003.
- Shewry, P. R., and S. Hey. 2015. Do “ancient” wheat species differ from modern bread wheat in their contents of bioactive components? Journal of Cereal Science 65:236–43. doi: 10.1016/j.jcs.2015.07.014.
- Shi, J., and M. Le Maguer. 2000. Lycopene in tomatoes: Chemical and physical properties affected by food processing. Critical Reviews in Food Science and Nutrition 40 (1):1–42. doi: 10.1080/10408690091189275.
- Shi, Z. 2015. Method for extracting carotenoid from waste oil sludge of ginkgo leaf extraction process. Patent No. CN104341332, filed October 24, 2014, and issued February 11, 2015.
- Shin, H. S., J. W. Kim, J. H. Kim, D. G. Lee, S. Lee, and D. Y. Kil. 2016. Effect of feeding duration of diets containing corn distillers dried grains with solubles on productive performance, egg quality, and lutein and zeaxanthin concentrations of egg yolk in laying hens. Poultry Science 95 (10):2366–71. doi: 10.3382/ps/pew127.
- Shulaev, V. 2006. Metabolomics technology and bioinformatics. Briefings in Bioinformatics 7 (2):128–39. doi: 10.1093/bib/bbl012.
- Sibeijn, M., J. H. Wolf, and A. Schaap. 2003. Isolation of cartenoid crystals. Patent No. US2003139480, July 29, 2002, and issued July 24, 2003.
- Simlat, M., P. Ślęzak, M. Moś, M. Warchoł, E. Skrzypek, and A. Ptak. 2016. The effect of light quality on seed germination, seedling growth and selected biochemical properties of Stevia rebaudiana Bertoni. Scientia Horticulturae 211:295–304. doi: 10.1016/j.scienta.2016.09.009.
- Singh, A., S. Ahmad, and A. Ahmad. 2015. Green extraction methods and environmental applications of carotenoids-a review. RSC Advances 5 (77):62358–93. doi: 10.1039/C5RA10243J.
- Smith, H. L., L. McAusland, and E. H. Murchie. 2017. Don’t ignore the green light: exploring diverse roles in plant processes. Journal of Experimental Botany 68 (9):2099–110. doi: 10.1093/jxb/erx098.
- Sommerburg, O., K. Meissner, M. Nelle, H. Lenhartz, and M. Leichsenring. 2000. Carotenoid supply in breast-fed and formula-fed neonates. European Journal of Pediatrics 159 (1–2):86–90. doi: 10.1007/PL00013811.
- Song, J., Q. Wei, X. Wang, D. Li, C. Liu, M. Zhang, and L. Meng. 2018. Degradation of carotenoids in dehydrated pumpkins as affected by different storage conditions. Food Research International (Ottawa, Ont.) 107:130–6. doi: 10.1016/j.foodres.2018.02.024.
- Souverein, O. W., J. H. M. de Vries, R. Freese, B. Watzl, A. Bub, E. R. Miller, J. J. M. Castenmiller, W. J. Pasman, K. van Het Hof, M. Chopra, et al. 2015. Prediction of fruit and vegetable intake from biomarkers using individual participant data of diet-controlled intervention studies. The British Journal of Nutrition 113 (9):1396–409. doi: 10.1017/S0007114515000355.
- Stinco, C. M., A. M. Benítez-González, D. Hernanz, I. M. Vicario, and A. J. Meléndez-Martínez. 2014. Development and validation of a Rapid Resolution Liquid Chromatography method for the screening of dietary plant isoprenoids: Carotenoids, tocopherols and chlorophylls. Journal of Chromatography A 1370:162–70. doi: 10.1016/j.chroma.2014.10.044.
- Stinco, C. M., A. M. Benítez-González, A. J. Meléndez-Martínez, D. Hernanz, and I. M. Vicario. 2018. Simultaneous determination of dietary isoprenoids (carotenoids, chlorophylls and tocopherols) in human faeces by Rapid Resolution Liquid Chromatography. Journal of Chromatography 1563:63–72.
- Stinco, C. M., M. L. Escudero-Gilete, F. J. Heredia, I. M. Vicario, and A. J. Meléndez-Martínez. 2016. Multivariate analyses of a wide selection of orange varieties based on carotenoid contents, color and in vitro antioxidant capacity. Food Research International (Ottawa, Ont.) 90:194–204. doi: 10.1016/j.foodres.2016.11.005.
- Stinco, C. M., J. Szczepańska, K. Marszałek, C. A. Pinto, R. S. Inácio, P. Mapelli-Brahm, F. J. Barba, J. M. Lorenzo, J. A. Saraiva, and A. J. Meléndez-Martínez. 2019. Effect of high-pressure processing on carotenoids profile, colour, microbial and enzymatic stability of cloudy carrot juice. Food Chemistry 299:125112. doi: 10.1016/j.foodchem.2019.125112.
- Strachan, D. B., A. Yang, and R. D. Dillon. 1993. Effect of grain feeding on fat colour and other carcass characteristics in previously grass-fed Bos indicus steers. Australian Journal of Experimental Agriculture 33 (3):269–73. doi: 10.1071/EA9930269.
- Strati, I. F., E. Gogou, and V. Oreopoulou. 2015. Enzyme and high pressure assisted extraction of carotenoids from tomato waste. Food and Bioproducts Processing 94:668–74. doi: 10.1016/j.fbp.2014.09.012.
- Strati, I. F., and V. Oreopoulou. 2011. Effect of extraction parameters on the carotenoid recovery from tomato waste. International Journal of Food Science & Technology 46 (1):23–9. doi: 10.1111/j.1365-2621.2010.02496.x.
- Strati, I. F., and V. Oreopoulou. 2014. Recovery of carotenoids from tomato processing by-products - A review. Food Research International 65:311–21. doi: 10.1016/j.foodres.2014.09.032.
- Suetsugu, N., and M. Wada. 2013. Evolution of three LOV blue light receptor families in green plants and photosynthetic stramenopiles: phototropin, ZTL/FKF1/LKP2 and aureochrome. Plant & Cell Physiology 54 (1):8–23. doi: 10.1093/pcp/pcs165.
- Sutthiwong, N., and L. Dufossé. 2014. Production of carotenoids by Arthrobacter arilaitensis strains isolated from smear-ripened cheeses. FEMS Microbiology Letters 360 (2):174–81. doi: 10.1111/1574-6968.12603.
- Swaminathan, S., and K. P. Madavalappil. 2008. Isolation and Purification of Carotenoids from Marigold Flowers. Patent No. US2008051591, filed April 25, 2005, and issued February 28, 2008.
- Takatani, N., T. Sawabe, T. Maoka, K. Miyashita, and M. Hosokawa. 2015. Structure of a novel monocyclic carotenoid, 3 ″-hydroxy-2′-isopentenylsaproxanthin ((3R, 2′ S)-2′-(3-hydroxy-3-methylbutyl)-3′, 4′-didehydro-1′, 2′-dihydro-β, ψ-carotene-3, 1′-diol), from a flavobacterium Gillisia limnaea strain DSM 15749. Biocatalysis and Agricultural Biotechnology 4 (2):174–9. doi: 10.1016/j.bcab.2015.02.005.
- Takemura, M., T. Maoka, A. Osawa, H. Higashinaka, H. Shimada, K. Shindo, and N. Misawa. 2015. (6E) and (6Z)-9′-Aporhodoxanthinone, novel carotenoids produced in zeaxanthin-synthesizing-Escherichia coli by redox stress. Tetrahedron Letters 56 (44):6063–5. doi: 10.1016/j.tetlet.2015.09.058.
- Talegawkar, S. A., E. J. Johnson, T. C. Carithers, H. A. Taylor, Jr, M. L. Bogle, and K. L. Tucker. 2008. Serum carotenoid and tocopherol concentrations vary by dietary pattern among African Americans. Journal of the American Dietetic Association 108 (12):2013–20. doi: 10.1016/j.jada.2008.09.004.
- Tanaka, M., Y. Kamiya, T. Suzuki, and Y. Nakai. 2010. Effect of citrus pulp silage feeding on concentration of beta-cryptoxanthin in plasma and milk of dairy cows. Animal Science Journal 81 (5):569–73. doi: 10.1111/j.1740-0929.2010.00775.x.
- Tangney, C. C., J. L. Bienias, D. A. Evans, and M. C. Morris. 2004. Reasonable estimates of serum vitamin E, vitamin C, and β-cryptoxanthin are obtained with a food frequency questionnaire in older black and white adults. The Journal of Nutrition 134 (4):927–34. doi: 10.1093/jn/134.4.927.
- Todd, G. N. 2000. High temperature countercurrent solvent extraction of capsicum solids. Patent No. US6074687, filed November 7, 1997, and issued June 13, 2000.
- Torregrosa, F., C. Cortés, M. J. Esteve, and A. Frígola. 2005. Effect of high-intensity pulsed electric fields processing and conventional heat treatment on orange-carrot juice carotenoids. Journal of Agricultural and Food Chemistry 53 (24):9519–25. doi: 10.1021/jf051171w.
- Treganowan, J., C. Santos, J. Kessler, D. Greenfell-Lee, J. Treganowan, C. Santos, J. Kessler, D. Grenfell-Lee, C. Toniato, and C. Schaefer. 2017. Process for isolating a carotenoid from a carotenoid-producing bioorganism. Patent No. US2017129854, filed March 26, 2015, and issued May 11, 2017.
- Tsushima, M., Y. Ikuno, S. Nagata, K. Kodama, and T. Matsuno. 2002. Comparative biochemical studies of carotenoids in catfishes. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 133 (3):331–6. doi: 10.1016/S1096-4959(02)00159-8.
- Tuan, P. A., A. A. Thwe, Y. B. Kim, J. K. Kim, S.-J. Kim, S. Lee, S.-O. Chung, and S. U. Park. 2013. Effects of white, blue, and red light-emitting diodes on carotenoid biosynthetic gene expression levels and carotenoid accumulation in sprouts of tartary buckwheat (Fagopyrum tataricum Gaertn.). Journal of Agricultural and Food Chemistry 61 (50):12356–61. doi: 10.1021/jf4039937.
- Tucker, K. L., H. Chen, S. Vogel, P. W. Wilson, E. J. Schaefer, and C. J. Lammi-Keefe. 1999. Carotenoid intakes, assessed by dietary questionnaire, are associated with plasma carotenoid concentrations in an elderly population. The Journal of Nutrition 129:438–45. doi:10.1093/jn/129.2.438
- Tugizimana, F., L. Piater, and I. Dubery. 2013. Plant metabolomics: A new frontier in phytochemical analysis. South African Journal of Science 109 (5–6):1–11. doi: 10.1590/sajs.2013/20120005.
- Tumbas Šaponjac, V., G. Ćetković, J. Čanadanović-Brunet, S. Djilas, B. Pajin, J. Petrović, S. Stajčić, and J. Vulić. 2017. Encapsulation of sour cherry pomace extract by freeze drying: Characterization and storage stability. Acta Chimica Slovenica 64 (2):283–9. doi: 10.17344/acsi.2016.2789.
- Turkiewicz, I. P., A. Wojdyło, K. Tkacz, and P. Nowicka. 2020. Carotenoids, chlorophylls, vitamin E and amino acid profile in fruits of nineteen Chaenomeles cultivars. Journal of Food Composition and Analysis 93:103608. doi: 10.1016/j.jfca.2020.103608.
- Tzanova, M., M. Argirova, and V. Atanasov. 2017. HPLC quantification of astaxanthin and canthaxanthin in salmonidae eggs. Biomedical Chromatography 31 (4):e3852. doi: 10.1002/bmc.3852.
- Unknown authors. 2013. Liquid formulations containing carotenoids. Patent No. JP5368316, filed January 15, 2008, and issued May 13, 2010.
- Unlu, N. Z., T. Bohn, D. M. Francis, H. N. Nagaraja, S. K. Clinton, and S. J. Schwartz. 2007. Lycopene from heat-induced cis-isomer-rich tomato sauce is more bioavailable than from all-trans-rich tomato sauce in human subjects. The British Journal of Nutrition 98 (1):140–6. doi: 10.1017/S0007114507685201.
- Usami, T., N. Mochizuki, M. Kondo, M. Nishimura, and A. Nagatani. 2004. Cryptochromes and phytochromes synergistically regulate Arabidopsis root greening under blue light. Plant & Cell Physiology 45 (12):1798–808. doi: 10.1093/pcp/pch205.
- USDA. 2010. National nutrient database for standard reference. US Department for Agriculture. www.ars.usda.gov/Service/docs.htm?docid=8964.
- Vági, E., B. Simándi, K. P. Vásárhelyiné, H. Daood, Á. Kéry, F. Doleschall, and B. Nagy. 2007. Supercritical carbon dioxide extraction of carotenoids, tocopherols and sitosterols from industrial tomato by-products. The Journal of Supercritical Fluids 40 (2):218–26. doi: 10.1016/j.supflu.2006.05.009.
- Van den Berg, H., R. Faulks, H. F. Granado, J. Hirschberg, B. Olmedilla, G. Sandmann, S. Southon, and W. Stahl. 2000. Review. The potential for the improvement of carotenoid levels in foods and the likely systemic effects. Journal of the Science of Food and Agriculture 80:880–912. doi:10.1002/(SICI)1097-0010(20000515)80:7<880::AID-JSFA646>3.0.CO;2-1
- Van Kappel, A. L., J. P. Steghens, A. Zeleniuch-Jacquotte, V. Chajès, P. Toniolo, and E. Riboli. 2001. Serum carotenoids as biomarkers of fruit and vegetable consumption in the New York Women’s Health Study. Public Health Nutrition 4 (3):829–35. doi: 10.1079/phn2000115.
- Van Keulen, F., A. L. Carolas, M. Lopes Brito, and B. Sommer Ferreira. 2010. Production of High-Purity Carotenoids by Fermenting Selected Bacterial Strains. Patent No. US2010145116, filed March 8, 2007, and issued June 10, 2010.
- Van Meulebroek, L., J. V. Bussche, K. Steppe, and L. Vanhaecke. 2014. High-resolution Orbitrap mass spectrometry for the analysis of carotenoids in tomato fruit: Validation and comparative evaluation towards UV-VIS and tandem mass spectrometry. Analytical and Bioanalytical Chemistry 406 (11):2613–26. doi: 10.1007/s00216-014-7654-6.
- Vargas-Murga, L., V. V. de Rosso, A. Z. Mercadante, and B. Olmedilla-Alonso. 2016. Fruits and vegetables in the Brazilian Household Budget Survey (2008–2009): carotenoid content and assessment of individual carotenoid intake. Journal of Food Composition and Analysis 50:88–96. doi: 10.1016/j.jfca.2016.05.012.
- Vashkevych, O. Y., Y. V. Stepnevcka, and I. V. Laptieva. 2010. Process for the production of carotenoid dye from plant raw material. Patent No. UA49676, filed October 29, 2009, and issued May 11, 2010.
- Vashkevych, O. Y., Y. V. Stepnevska, and R. R. Yudych. 2012. Process for the preparation of alcohol-water soluble carotenoid pigment of plant raw material. Patent No. UA69607, filed September 20, 2011, and issued May 10, 2012.
- Veberic, R., J. Jurhar, M. Mikulic-Petkovsek, F. Stampar, and V. Schmitzer. 2010. Comparative study of primary and secondary metabolites in 11 cultivars of persimmon fruit (Diospyros kaki L.). Food Chemistry 119 (2):477–83. doi: 10.1016/j.foodchem.2009.06.044.
- Velázquez-Estrada, R. M., M. M. Hernández-Herrero, C. E. Rüfer, B. Guamis-López, and A. X. Roig-Sagués. 2013. Influence of ultra high pressure homogenization processing on bioactive compounds and antioxidant activity of orange juice. Innovative Food Science & Emerging Technologies 18:89–94. doi: 10.1016/j.ifset.2013.02.005.
- Vervoort, L., I. Van Der Plancken, T. Grauwet, P. Verlinde, A. Matser, M. Hendrickx, and A. Van Loey. 2012. Thermal versus high pressure processing of carrots: A comparative pilot-scale study on equivalent basis. Innovative Food Science & Emerging Technologies 15:1–13. doi: 10.1016/j.ifset.2012.02.009.
- Viuda-Martos, M., E. Sanchez-Zapata, E. Sayas-Barberá, E. Sendra, J. A. Pérez-Álvarez, and J. Fernández-López. 2014. Tomato and tomato byproducts. Human health benefits of lycopene and its application to meat products: A review. Critical Reviews in Food Science and Nutrition 54 (8):1032–49. doi: 10.1080/10408398.2011.623799.
- Wang, J. L. 2007. Method for improving carotenoid output by adding glycerol in ferment process. Patent No. CN101041848, filed April 23, 2007, and issued September 26, 2007.
- Wang, S., S. Errington, and H. H. Yap. 2008, September. Studies on carotenoids from lupin seeds. In Lupins for Health and Wealth’Proceedings of the 12th International Lupin Conference, 14–8.
- Wang, Y., and K. M. Folta. 2013. Contributions of green light to plant growth and development. American Journal of Botany 100 (1):70–8. doi: 10.3732/ajb.1200354.
- Wawrzyniak, A., J. Hamułka, E. Friberg, and A. Wolk. 2013. Dietary, anthropometric, and lifestyle correlates of serum carotenoids in postmenopausal women. European Journal of Nutrition 52 (8):1919–26. doi: 10.1007/s00394-013-0493-y.
- Wei, D., and C. Ma. 2014. Method for extracting nutrients from spirulina in grading manner. Patent No. CN104086649, filed July 8, 2014, and issued October 8, 2014.
- Weller, P., and D. E. Breithaupt. 2003. Identification and quantification of zeaxanthin esters in plants using liquid chromatography-mass spectrometry. Journal of Agricultural and Food Chemistry 51 (24):7044–9. doi: 10.1021/jf034803s.
- Wen, X., J. Hempel, R. M. Schweiggert, Y. Ni, and R. Carle. 2017. Carotenoids and carotenoid esters of red and yellow Physalis (Physalis alkekengi L. and P. pubescens L.) fruits and calyces. Journal of Agricultural and Food Chemistry 65 (30):6140–51. doi: 10.1021/acs.jafc.7b02514.
- Willett, W., J. Rockström, B. Loken, M. Springmann, T. Lang, S. Vermeulen, T. Garnett, D. Tilman, F. DeClerck, A. Wood, et al. 2019. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet (London, England) 393 (10170):447–92. doi: 10.1016/S0140-6736(18)31788-4.
- Whitelam, G., and K. Halliday. 2007. Light and plant development. Oxford, UK: Blackwell Publishing.
- Wu, W., J. Wang, W. Xu, and X. Zhang. 2013. Novel process for high-effective extraction of carotenoid in Blakeslea trispora. Patent No. CN103012230, filed January 6, 2013, and issued April 3, 2013.
- Xie, X., X. Lu, L. Wang, L. He, and G. Wang. 2020. High light intensity increases the concentrations of β-carotene and zeaxanthin in marine red macroalgae. Algal Research 47:101852. doi: 10.1016/j.algal.2020.101852.
- Xinde, C. X., X. Xu, B. Chen, D. Zhou, S. Ye, S. Huang, and B. Shao. 2006. Method for separating and purifying fatty acid ester of lutein in high content from resin of marigold oil. Patent No. CN1872839, filed May 17, 2006, and issued December 6, 2006.
- Xingjun, W., J. Nana, L. Havel, and Z. Chuanzhi. 2011. Method for improving carotenoid content of tomato. Patent No. CN101979601, filed September 21, 2010, and issued February 23, 2011.
- Xiuying, K., X. Yong, L. Limin, and D. Yangji. 2010. Method for extracting carotenoid from Chinese wolfberry by microwaves. Patent No. CN101898989, filed July 21, 2010, and issued December 1, 2010.
- Xu, D. P., Y. Li, X. Meng, T. Zhou, Y. Zhou, J. Zheng, J. J. Zhang, and H. B. Li. 2017. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. International Journal of Molecular Sciences 18 (1):96. doi: 10.3390/ijms18010096.
- Yabuta S., M.Urata R. Y.Wai Kun M.Masaki, and Y. Shidoji. 2016. Common SNP rs6564851 in the BCO1 gene affects the circulating levels of β-carotene and the daily intake of carotenoids in healthy Japanese women. PLoS One 11 (12):e0168857. doi: 10.1371/journal.pone.0168857.
- Yabuzaki, J. 2017. Carotenoids Database: Structures, chemical fingerprints and distribution among organisms. Database 2017 (1):1–11. doi: 10.1093/database/bax004.
- Yang, A., M. J. Brewster, M. G. Lanari, and R. K. Tume. 2002. Effect of vitamin E supplementation on alpha-tocopherol and beta-carotene concentrations in tissues from pasture- and grain-fed cattle. Meat Science 60 (1):35–40. doi: 10.1016/S0309-1740(01)00102-4.
- Yang, A., T. W. Larsen, and R. K. Tume. 1992. Carotenoid and retinol concentrations in serum, adipose-tissue and liver and carotenoid transport in sheep, goats and cattle. Australian Journal of Agricultural Research 43 (8):1809–17. doi: 10.1071/AR9921809.
- Yi, X., F. Zhang, W. Xu, J. Li, W. Zhang, and K. Mai. 2014. Effects of dietary lipid content on growth, body composition and pigmentation of large yellow croaker Larimichthys croceus. Aquaculture 434:355–61. doi: 10.1016/j.aquaculture.2014.08.035.
- Yong, L. C., M. R. Forman, G. R. Beecher, B. I. Graubard, W. S. Campbell, M. E. Reichman, P. R. Taylor, E. Lanza, J. M. Holden, and J. T. Judd. 1994. Relationship between dietary intake and plasma concentrations of carotenoids in premenopausal women: Application of the USDA-NCI carotenoid food-composition database. The American Journal of Clinical Nutrition 60 (2):223–30. doi: 10.1093/ajcn/60.2.223.
- Yoo, H. S. 2006. Method for directly extracting lycopen from tomato and extract including lycopen using the method. Patent No. KR20060112346, filed 26 April, 2005, and issued November 1, 2006.
- Young, A. J. 1993. Occurrence and distribution of carotenoids in photosynthetic systems. In Carotenoids in photosynthesis, eds. A. Young and G. Britton, 16–71. London, UK: Chapman and Hall.
- Yuan, B., and Y. Fan. 2016. Refined apricot kernel oil and preparing method thereof. Patent No. CN105767233, filed April 13, 2016, and issued July 20, 2016.
- Yuan, Z., L. Deng, B. Yin, S. Yao, and K. Zeng. 2017. Effects of blue LED light irradiation on pigment metabolism of ethephon-degreened mandarin fruit. Postharvest Biology and Technology 134:45–54. doi: 10.1016/j.postharvbio.2017.08.005.
- Yuejin, H., C. Jianhui, W. Hu, and T. Bing. 2012. Method for producing natural carotenoid by cultivating deinococcus radiodurans. Patent No. CN102559827, filed January 4, 2012, and issued July 11, 2012.
- Yueqi, G., H. Jiangong, L. Ning, L. Xiaolu, L. Yang, L. Gangsan, R. Fengzhi, S. Fei, W. Haiyan, W. Jian, et al. 2010. Method for separating and purifying all-trans xanthophyl powder. Patent No. CN101838229, filed March 19, 2009, and issued September 22, 2010.
- Zaťková, I., M. Sergejevová, J. Urban, R. Vachta, D. Štys, and J. Masojídek. 2011. Carotenoid-enriched microalgal biomass as feed supplement for freshwater ornamentals: Albinic form of Wels catfish (Silurus glanis). Aquaculture Nutrition 17 (3):278–86. doi: 10.1111/j.1365-2095.2009.00751.x.
- Zawadzki, F., I. N. do Prado, and S. Prache. 2013. Influence of level of barley supplementation on plasma carotenoid content and fat spectrocolorimetric characteristics in lambs fed a carotenoid-rich diet. Meat Science 94 (3):297–303. doi: 10.1016/j.meatsci.2013.03.004.
- Zhai, S. N., X. C. Xia, and Z. H. He. 2016. Carotenoids in staple cereals: Metabolism, regulation, and genetic manipulation. Frontiers in Plant Science 7:1197. doi: 10.3389/fpls.2016.01197.
- Zhang, L., G. Ma, K. Yamawaki, Y. Ikoma, H. Matsumoto, T. Yoshioka, S. Ohta, and M. Kato. 2015. Effect of blue LED light intensity on carotenoid accumulation in citrus juice sacs. Journal of Plant Physiology 188:58–63. doi: 10.1016/j.jplph.2015.09.006.
- Zhang, R., N. Lebovka, L. Marchal, E. Vorobiev, and N. Grimi. 2020. Multistage aqueous and non-aqueous extraction of bio-molecules from microalga Phaeodactylum tricornutum. Innovative Food Science & Emerging Technologies 62:102367. doi: 10.1016/j.ifset.2020.102367.
- Zhang, X., A. Ke, N. Li, X. Li, F. Ren, S. Chen, H. Wang, X. Cheng, Y. Lin, L. Li, et al. 2012. Method for separating and purifying all-trans high-purity lutein esters powder. Patent No. CN102838519, filed March 13, 2012, and issued December 26, 2012.
- Zhao, Z. 2013. Method for extracting seabuckthorn seeds by using supercritical CO2. Patent No. CN102994217, filed November 15, 2012, and issued March 27, 2013.
- Zhen, H., M. Qing, and Y. Meijing. 2011. Method for extracting carotenoid from physalis persistent calyx. Patent No. CN101973920, filed October 18, 2010, and issued February 16, 2011.
- Zhirong, C., C. Jianfeng, Y. Hong, Z. Hong, C. Dan, S. Lifang, L. Jiandong, C. Guangwen, and S. Lei. 2009. Method of preparing nano-dispersed high-all-trans-carotenoid microcapsules. Patent No. CN101549273, filed March 30, 2009, and issued October 7, 2009.
- Zhong, L., K.-E. Gustavsson, S. Oredsson, B. Głąb, J. Lindberg Yilmaz, and M. E. Olsson. 2016. Determination of free and esterified carotenoid composition in rose hip fruit by HPLC-DAD-APCI(+)-MS. Food Chemistry 210:541–50. doi: 10.1016/j.foodchem.2016.05.002.
- Zhou, C., D. Zhao, Y. Sheng, J. Tao, and Y. Yang. 2011. Carotenoids in fruits of different persimmon cultivars. Molecules (Basel, Switzerland) 16 (1):624–36. doi: 10.3390/molecules16010624.
- Zhou, L., and X. Ding. 2014. Culture method of high-yield carotenoid cordyceps militaris. Patent No. CN104004815, filed May 29, 2014, and issued August 27, 2014.
- Zhou, W., Y. Niu, X. Ding, S. Zhao, Y. Li, G. Fan, S. Zhang, and K. Liao. 2020. Analysis of carotenoid content and diversity in apricots (Prunus armeniaca L.) grown in China. Food Chemistry 330:127223. doi: 10.1016/j.foodchem.2020.127223.
- Ziegler, J. U., S. Wahl, T. Wurschum, C. F. Longin, R. Carle, and R. M. Schweiggert. 2015. Lutein and lutein esters in whole grain flours made from 75 genotypes of 5 Triticum species grown at multiple sites. Journal of Agricultural and Food Chemistry 63 (20):5061–71. doi: 10.1021/acs.jafc.5b01477.
- Zoccali, M., D. Giuffrida, P. Dugo, and L. Mondello. 2017. Direct online extraction and determination by supercritical fluid extraction with chromatography and mass spectrometry of targeted carotenoids from red Habanero peppers (Capsicum chinense Jacq.). Journal of Separation Science 40 (19):3905–13. doi: 10.1002/jssc.201700669.