Abstract
Obesity is a mostly preventable diet-related disease and currently a major challenge for human populations worldwide. Obesity is a major risk factor for diseases such as type 2 diabetes mellitus (T2DM), cardiovascular disease (CVD) and certain cancers. Dietary fiber is a complex mixture of non-digestible molecules, mostly polysaccharides. Multiple epidemiological studies have demonstrated statistically significant reductions in risks of obesity, T2DM, CVD, colorectal cancer, and pre-menopausal breast cancer with higher dietary fiber intakes. Various direct and indirect mechanisms have been proposed including altered digestion and absorption, stimulation of gut hormones including glucagon-like-peptide-1 (GLP-1) and peptide YY (PYY), reduced appetite, and altered metabolism of bile and cholesterol. These may act via pathways involving G-protein-coupled receptors (GPRs), histone deacetylase (HDAC), and aromatase enzymes. Ultimately, fiber intake contributes to improving glucose levels and insulin sensitivity, lowering risk of T2DM, CVD and certain cancers. Therefore, diets rich in dietary fiber should be encouraged to prevent obesity and associated chronic disease.
Introduction – Dietary fiber definition and its constituents
Diet plays a major role in the prevention of obesity and associated non-communicable diseases such as type 2 diabetes mellitus (T2DM), cardiovascular disease (CVD) and certain cancers World Health Organization (World Health Organization (WHO) Citation2019), such as colon cancer and breast cancer (National Cancer Institute Citation2017; National Institute of Diabetes and Digestive and Kidney Diseases Citation2015). There is a range of evidence from epidemiology to clinical intervention trials that suggests that dietary fiber intake lowers the risk of obesity and its related non-communicable diseases. A major challenge in reviewing the evidence is the complexity of dietary fiber components and its dietary sources. Different countries and scientific disciplines have adopted different definitions, which complicates comparison between studies.
There has been an evolution in the definition of dietary fiber, and the methodology used to analyze it for food composition data. In the UK, the British Nutrition Foundation (BNF) defines dietary fiber as “plant-based carbohydrates that, unlike other carbohydrates (such as sugars and starch), are not digested in the small intestine and so reach the large intestine or colon” (British Nutrition Foundation Citation2018). The European Union’s (EU) define fiber “as carbohydrate polymers with three or more monomeric units, which are neither digested nor absorbed in the human small intestine” (European Commission Citation2020). Food Standards Australia New Zealand define dietary fiber as the “fraction of the edible parts of plants or their extracts, or synthetic analogues, that are resistant to the digestion and absorption in the small intestine, usually with complete or partial fermentation in the large intestine” (Australian Government National Health and Medical Research Council Citation2019). This definition includes polysaccharides, oligosaccharides and lignins (Australian Government National Health and Medical Research Council Citation2019). The US Food and Drug Administration (FDA) defines dietary fiber as “non-digestible soluble and insoluble carbohydrates, with three or more monomeric units and lignin, that are intrinsic and intact in plants; isolated or synthetic non-digestible carbohydrates, with three or more monomeric units, determined by the FDA to have physiological effects that are beneficial to human health. Such effects include lowering blood pressure, lowering blood glucose, lowering blood cholesterol levels, increased bowel movement frequency (improved laxation), increased mineral absorption in the intestinal tract, and reduced energy intake (for example, due to fiber promoting feelings of fullness)”. Finally, CODEX Alimentarius defines dietary fiber as “carbohydrate polymers with ten or more monomeric units, which are not hydrolyzed by the endogenous enzymes in the small intestine of humans, and belong to one of the following categories: 1) edible carbohydrate polymers naturally occurring in the food as consumed, 2) carbohydrate polymers which have been obtained from food raw material by physical, enzymatic or chemical means, and which have been shown to have a physiological effect of benefit to health as demonstrated by generally accepted scientific evidence to competent authorities, 3) synthetic carbohydrate polymers, which have been shown to have a physiological effect of benefit to health as demonstrated by generally accepted scientific evidence to competent authorities”. Overall, each of these definitions are similar in that they depict dietary fiber as generally originating from plant-based sources, are not digested by human enzymes or absorbed in the small intestine, and contribute positively to human health.
Furthermore, dietary fiber is often described according to its solubility in water. As such, dietary fiber can be classified as soluble or insoluble fiber. Soluble dietary fiber can hydrate in water, forming a gel-like substance within the gastrointestinal (GI) tract which increases the viscosity of GI contents and altering transit time, digestion and absorption of GI contents (Cameron-Smith, Collier, and O’Dea Citation1994). Soluble fibers include several types of polysaccharide including hemicelluloses (e.g., xyloglucans in fruits, vegetables and some non-cereal seeds, β-glucans in oats and barley, arabinoxylans in wheat and rice) and pectins (Evans Citation2020), which can be found abundantly in foods such as legumes, fruits and vegetables (Dhingra et al. Citation2012; Nakashima et al. Citation2018; Ridley, O’Neill, and Mohnen Citation2001). Soluble fiber also includes algae polysaccharides such as alginate and carrageenan, and gums such as guar gum and gum arabic. In the more recent US definition of dietary fiber, soluble oligosaccharides such as inulins and raffinose-like oligosaccharides would also be included. Insoluble dietary fiber, on the other hand, is the fiber that cannot dissolve or is poorly hydrated in water (Stephen et al. Citation2017). This type of fiber increases faecal bulk (Eggum Citation1995), and decreases transit time (Roberfroid Citation1993). Insoluble fibers are comprised primarily of cellulose, some hemicelluloses and lignins (O’Grady, O’Connor, and Shanahan Citation2019). Insoluble fiber is the most abundant type of fiber in most foods including legumes, fruits and vegetables, and is highly dominant in whole grains such as wheat, oat, barley and rye (British Nutrition Foundation Citation2018; Oldways Whole Grains Council Citation2020; Lunn and Buttriss Citation2007). The solubility of fiber is likely to impact on its fermentability, and therefore has physiological relevance. Assessment of fiber dietary intake remains a challenge. New tools are being developed to better assess fiber intake (Rijnaarts et al. Citation2021).
The current dietary guidelines in the UK for dietary fiber intake is 30 g/day AOAC for both females and males aged 16 to 64-years-old (British Nutrition Foundation Citation2018; Public Health England Citation2016). However, the average UK population is not currently achieving their recommended dietary fiber intake. According to the 2014–2016 National Diet and Nutrition Survey (NDNS) by Public Health England (PHE), the average dietary fiber intake of 19 to 64-year-olds was 19 g/day AOAC, with intakes of 20.7 g/day AOAC and 17.4 g/day AOAC for men and women, respectively (Public Health England Citation2018). Most food-based dietary guidelines recommend consuming at least 5 portions of fruits or vegetables (current average UK intake is just above 2), cereals in wholegrain form (e.g., pasta, bread, noodles) and increase intake of legumes, nuts and seeds (Gressier and Frost Citation2022). Major efforts in industry reformulation and the current drive toward sustainable plant-based diets should assist in increasing fiber intakes, though long term epidemiological trials are needed to evaluate the effects.
Obesity is one of the biggest and most serious public health issues currently facing the human population (World Obesity Citation2019; Krzysztoszek, Laudańska-Krzemińska, and Bronikowski Citation2019; Lee Citation2020). A third of the global population is now considered to be overweight or obese, a figure which has doubled since the 1980s (Chooi, Ding, and Magkos Citation2019). An individual is considered obese when their body mass index (BMI) is > 30 kg/m2 (30–39.9 kg/m2) and severely obese when their BMI is > 40 kg/m2 (NHS Citation2019; Beuther and Sutherland Citation2007). The World Health Organization stated that 39% of adults were classified as overweight and 13% as obese in 2016 (WHO Citation2020). Projections to 2030 predict that 20% of the global adult population, estimated to be around 573 million people will be obese (Kelly et al. Citation2008). Worldwide obesity has also been seen to increase within children and adolescents. In 1975, less than 4% of children and adolescents were classified as overweight or obese. By 2016, this figure rose to 18% (WHO 2020). Furthermore, The Global Burden of Disease Study stated that, in 2017, obesity was the major cause of approximately 4.7 million premature deaths worldwide (Stanaway et al. Citation2018).
The etiology of obesity is complex. Typically, obesity develops as a result of an unhealthy high-energy diet (Swinburn et al. Citation2011), together with other lifestyle factors such as low physical activity (NHS Citation2019; Milagro, Moreno-Alaga, and Martinez Citation2020). The so called “Western” diet is a prime example of an unhealthy diet that is driving obesity and chronic disease (Manzel et al. Citation2014). Unhealthy diets are characterized by large portion sizes, and consumption of red and processed meats, sugar sweetened beverages, and foods high in saturated fats, added sugars, refined carbohydrates and salt (Manzel et al. Citation2014; Hariharan, Vellanki, and Kramer Citation2015), while often lacking in fiber-rich foods such as fruits, vegetables, beans, legumes, nuts, seeds, and whole grains (Harvard Citation2020; Birchenough et al. Citation2019; Chan et al. Citation2019). It must be noted that unhealthy dietary patterns are increasingly common across the world, and efforts to measure the extent of the nutrition transition at global level is ongoing (Singh et al. Citation2020). On the other hand, healthy dietary patterns to reduce obesity across geographies are also being proposed (Diotallevi et al. Citation2020).
Interventions to prevent or reverse obesity commonly involve dietary advice that promotes diverse diets containing a high proportion of fruits, vegetables, and other fiber-rich foods, in combination with regular physical activity (Ofei Citation2005). Obesity prevention is extremely important to decrease the risk of overall mortality (Thorpe and Ferraro Citation2004), and also because obesity is a major risk factor for many non-communicable diseases such as T2DM, CVD, and certain cancers (Nyberg et al. Citation2018; Poirier et al. Citation2006), including colorectal cancer and breast cancer (National Cancer Institute Citation2017; National Institute of Diabetes and Digestive and Kidney Diseases Citation2015; Sung et al. Citation2011).
This review evaluates the epidemiological and mechanistic evidence relating to dietary fiber and the prevention of obesity and its related diseases. We focus on the overall effect of dietary fiber, rather than on individual fiber components.
Epidemiological evidence linking fiber intake to the prevention of obesity and obesity-related diseases
Fiber in the prevention of obesity
The role of dietary fiber in obesity prevention has been the subject of systematic reviews and meta-analyses. The meta-analysis conducted by Chen et al. (Citation2017) examined 14 studies (n = 11 cross-sectional studies, n = 3 cohort studies). After removing 4 studies that showed high heterogeneity and publication bias, the analysis showed a significant protective effect of dietary fiber against metabolic syndrome risk. However, the authors concluded that there were insufficient high quality cohort studies to support the observations. Systematic review and meta-analysis of 15 viscous fiber RCTs was carried out by Jovanovski et al. (Citation2021). The analysis suggested significant effects of viscous fiber on body weight, percentage body fat and BMI, but not weight circumference. It is clear that the effects depend on the type of fiber and the host effects. For example, a Dutch study conducted by van de Vijver et al. (Citation2009). This study included male (n = 2,078) and female (n = 2,159) participants, aged 55 to 69-years-old, randomly selected from the Netherlands Cohort Study (NLCS) cohort. The study showed that there was a significant inverse association between wholegrain intake and BMI, as well as risk of overweight and obesity, in both men and women. However, when focusing on fiber intake, a difference between sexes was observed. For men, BMI decreased significantly by 0.04 kg/m2 (p < 0.01) and 0.29 kg/m2 (p < 0.01) for every 1 g/day and 1 g/MJ increase in fiber, respectively. No significant effect was observed for women. This was attributed by the authors as reporting bias, whereby women tended to under report energy dense foods which then leads to over reporting of fiber intake. A study involving Japanese women did show reduced BMIs with increased fiber consumption (Murakami et al. Citation2007). The cross-sectional study consisted of 3,931 Japanese female dietetics students aged 18 to 20-years-old. Self-administered questionnaires were used to collect information relating to dietary and lifestyle factors within the previous month. The study showed that higher fiber intakes were associated with lower BMI, with a difference of 0.6 kg/m2 for participants consuming 4.3 g/4,186 kJ versus 9.0 g/4,186 kJ (p < 0.0001). Similar differences in BMI were observed with soluble and insoluble fiber consumption. Increases in soluble fiber from 1.1 g/4,186 kJ to 2.4 g/4,186 kJ and insoluble fiber from 3.2 g/4,186 kJ to 6.5 g/4,186 kJ were associated with a lower BMI of 0.8 and 0.6 kg/m2 (p < 0.0001 for both), respectively. However, one study on a Belgian population (n = 3,083) found no association between BMI and fiber intakes but did find a statistically significant reduction in waist circumference (WC) with higher fiber intake (p < 0.0001) (Lin et al. Citation2011). In this study, gender differences in fiber intake were observed and found that gender, as well as geographical location, age and level of education, were confounding factors for the effects of fiber on measures of obesity. Overall, these findings support the hypothesis that higher dietary fiber intakes are associated with lower incidence of overweight and/or obesity, but biological and non-biological influencing factors need to be further explored.
Fiber in the prevention of T2DM
Meta-analyses have evaluated the role of fiber in T2DM prevention. One meta-analysis of 15 RCTs concluded that dietary fiber intake, through high-fiber diets or diet supplements, significantly improved several biomarkers of T2DM including fasting blood glucose and HbA1c (Post et al. Citation2012). Meanwhile, a meta-analysis of 23 prospective cohort studies investigated the effect of fruit and vegetable consumption on diabetes risk. It concluded that the effect may be partially attributed to fiber content in these foods (Wang et al. Citation2016). A meta-analysis also concluded that intake of wholegrains improved glycemic markers including fasting glucose, glycated hemoglobin (Hb1Ac) and insulin sensitivity (HOMA-IR) (Li et al. Citation2021). Interestingly, the study showed a higher effect of oat consumption compared to wheat and rice. Most meta-analyses show high degree of heterogeneity attributed to differences in food sources, doses and populations.
The EPIC-InterAct study conducted by The InterAct Consortium (Citation2015) examined the link between dietary fiber intake and the incidence of T2DM in eight European countries including Germany, Denmark, Italy, France, the Netherlands, the UK, Sweden and Spain. The study involved a nested case-cohort made up of 26,088 participants obtained from the European Prospective Investigation into Cancer and Nutrition (EPIC) study cohort. The 26,088-participant cohort was made up of two groups, the sub-cohort (n = 15,258) and the incident T2DM cases (n = 11,559), with 729 of the T2DM incidence cases coming from the sub-cohort group. The results of the study showed statistically significant reductions in T2DM risk with total fiber consumption, vegetable fiber consumption and cereal fiber consumption, when adjusted for dietary and lifestyle factors. When total fiber consumption was compared between a high intake (> 26.4 g/day, median 29.7 g/day) and low intake (< 18.9 g/day, median 16.3 g/day), a reduced relative risk of T2DM of 18% was calculated (p = 0.02). With vegetable fiber, an intake of over 5.3 g/day (median 6.9 g/day) was estimated to significantly reduce T2DM risk by 16% when compared to daily intakes of less than 2.4 g/day (median 1.4 g/day) (p < 0.01). With regards to cereal fiber, a 19% reduced risk of T2DM was ascertained for a consumption of over 10.9 g/day (median 13.7 g/day) compared to the lower consumption of less than 5.7 g/day (median 4.3 g/day) (p < 0.01). When fruit fiber consumption was analyzed, no significant reductions in T2DM risk were observed between the lower intake of less than 2.3 g/day (median 1.4 g/day) and higher intake of more than 6.3 g/day (median 8.4 g/day) (p = 0.74). However, after adjusting for BMI, none of these results for total fiber, vegetable fiber or cereal fiber were found to be statistically significant. A study conducted by Montonen et al. (Citation2003) also showed statistically significant reductions in T2DM risk with higher consumptions of total fiber and cereal fiber when comparing higher and lower consumptions. The study was based in Finland and involved a cohort of 4,316 participants (males n = 2,286 and females n = 2,030) aged 40 to 69-years-old, similar to that of the EPIC-InterAct study cohort. None of the participants had diabetes at baseline. Like the previous study, a 10-year follow-up was done to determine the number of T2DM incidence using the nationwide register. This revealed 54 and 102 T2DM cases within the men and women, respectively. The results of the study showed a reduced risk of T2DM by 49% with the higher fiber intake quartile (33.2–118 g/day) compared to the lower intake quartile (2.6–19.2 g/day) (p = 0.04). However, it must be noted that the fiber intake ranges are very large. With regards to cereal fiber, a statistically significant reduction in T2DM risk by 61% was obtained when comparing higher and lower intakes (p = 0.01). This study showed no significant reduction in T2DM risk with fruit or vegetable fiber intake when comparing the lower intakes. The risk reductions were more pronounce in obese and older sub-sets of the cohort. It is not completely clear why no significant associations have been made between fruit/vegetable fiber and T2DM risk reduction, but have been made with cereal fiber. One explanation may be related the amounts of these foods that are habitually consumed. For example, in Montonen et al. (Citation2003) Finnish study, participants with high intakes of cereal fiber were consuming 67.8 g/day on average compared to average fruit and vegetable fiber intakes of 3.3 g/day and 6.5 g/day, respectively. Therefore, stronger associations may be observed between cereal fiber and T2DM risk compared to fruit and vegetable fiber. The amounts of certain foods and fiber consumed in this study are much larger than in other studies and may therefore not be applicable to the other populations.
Fiber in the prevention of CVD
Several systematic reviews have investigated the effect of fiber intake on CVD risk. Systematic review and meta-analysis of 22 cohort studies showed a protective effect of dietary fiber on both CVD and coronary heart disease (Threapleton et al. Citation2013). Interestingly, cereal and vegetable fiber were associated with reduced risk of CVD and coronary heart disease, while fruit fiber was only associated with reduced risk of CVD. Oat has received particular attention due to the putative effects of beta-glucan on cholesterol. A meta-analysis of 8 prospective cohort studies showed a protective effect of oat consumption on T2DM and overall mortality risk, but not consistently with CVD risk (Wehrli et al. Citation2021). A systematic review Martínez-González et al. (Citation2002) showed the potential role of dietary fiber in the prevention of CVD, specifically acute myocardial infarction (AMI). The study was a case-control study based within three university hospitals in Pamplona, Spain. Cases (n = 171, mean age 61.7-years-old) were matched with controls (n = 171, mean age 61.4-years-old) of the same age, gender and who were admitted to the same university hospital. In order to obtain dietary intake information, FFQs consisting of 136 different food items were completed by each participant. From which, dietary fiber intakes were split into five quintiles ranging from Q1, the lowest dietary fiber intake (median 19 g/day), to Q5, the highest dietary fiber intake (median 45 g/day). After analysis of the dietary intake data and adjustment for dietary and lifestyle factors, statistically significant reductions in first AMIs were observed, with increases in prevention as dietary fiber intake increased. Q1 (19 g/day) was used as the reference, so no reduced risk was obtained with this amount of fiber. The next four quintiles, Q2 (25 g/day), Q3 (29 g/day), Q4 (35 g/day) and Q5 (45 g/day) showed reduced risks of first AMI by 49%, 79%, 74% and 86%, respectively (test for trend p = 0.01) when comparing each intake quintile to Q1. This reduced risk of CVD with increased total fiber consumption supported the findings by Bazzano et al. (Citation2003). In that prospective cohort study which included 9,776 American adults aged 25 to 74-years-old who had no history of CVD at baseline. In order to obtain dietary data, 24 hr recalls were conducted at baseline. During an average follow-up of 19 years, cardiovascular events such as coronary heart disease (CHD) and stroke, and CVD incidence were determined from participant medical records and or death certificates. The results of the study suggest that higher intakes of total dietary fiber are beneficial to reduce the risk of CHD and CVD incidence significantly, when comparing the highest intakes (> 15.9 g/1,735 kcal) to the lowest intakes (< 7.7 g/1,735 kcal). For CHD, the higher total dietary fiber intake, compared to lower intake, reduced a person’s risk of incidence by 12% (p for trend = 0.05). For CVD incidence, the higher fiber intake reduced a person’s risk by 11% (p for trend = 0.01). Both these statistically significant results were obtained after adjustments for various potential lifestyle and dietary confounders. This study further looked at soluble dietary fiber specifically, in terms of reduced CVD and cardiovascular event risk. The results showed that higher intakes of soluble fiber (> 4.0 g/1,735 kcal) compared to lower intakes (< 1.3 g/1,735 kcal) produced statistically significant reduced risks of CHD mortality, CVD incidence and CVD mortality by 24% (p for trend = 0.01), 10% (p for trend = 0.01) and 12% (p for trend = 0.03), respectively. Reduced risk of a similar magnitude as seen here with CVD and cardiovascular events were identified within cancer prevention with dietary fiber intake (Dahm et al. Citation2010).
Fiber in the prevention of colorectal and breast cancer
Systematic reviews and meta-analyses have also demonstrated reductions in the risk of both colorectal and breast cancer with higher fiber intakes. Systematic review and dose-response meta-analysis of 25 prospective studies found a dose-dependent effect of dietary fiber, particularly cereal fiber and whole grains, in reducing the risk of colorectal cancer (RR 0.83 with an increment of three servings). The effects were weaker for total, vegetable and fruit fibers (Aune et al. Citation2011). The study by Dahm et al. (Citation2010) followed a prospective nested case-control study design within seven different UK cohort studies. The study involved 579 case patients who had developed colorectal cancer and 1,996 matched control participants, aged 43 to 71.9-years-old. Dietary data was collected from each participant (n = 2,575) via food diaries and FFQs. This dietary data from both dietary methods was used to calculate the risks of developing colorectal, rectal and colon cancers. When analysis was conducted using the food diary data, the highest fiber intake (mean 24.5 g/day) compared to the lowest reference intake (mean 8.9 g/day) showed reduced risks of colon and rectal combined, colon and rectal cancers by 27%, 30% and 18%, respectively. However, none of these values were statistically significant when p for trend was tested (all p > 0.05). Nevertheless, analysis was also conducted on dietary fiber intake density, that is the amount of fiber per MJ of energy, to assess reduced risks of these cancers. For both colon and rectal combined, and colon cancer, statistically significant reductions of 34% (p for trend = 0.012) and 40% (p for trend = 0.014) were identified, respectively, when the highest (mean 3.0 g/MJ) and lowest (mean 1.2 g/MJ) intake densities were compared. While this analysis was also done for rectal cancer and showed a reduced risk of 16% between the highest and lowest densities, it was not statistically significant (p for trend = 0.40).
These findings were coherent with those found by Wakai et al. (Citation2007) as reduced risks were seen for colorectal and colon cancer but not for rectal cancer alone. Wakai et al. (Citation2007) conducted a cohort study in Japan consisting of 43,115 men and women aged between 40- and 79-years-old. Dietary data was also obtained through FFQs, from which dietary fiber intakes were calculated. An average follow-up of 7.6 years took place and from this, together with the dietary data, risks for developing colorectal, colon and rectal cancers were determined. When comparing the highest fiber intake (mean 13.4 g/day) to the lowest intake (mean 7.1 g/day) reduced risks of 27% (p for trend = 0.028) and 42% (p for trend = 0.002) for colorectal cancer and colon cancer were obtained. For rectal cancer, no statistically significant reduction in risk was observed. Therefore, corresponds to the results of Dahm et al. (Citation2010) study and provides evidence for a link between fiber and lower colorectal cancer risk.
Wakai et al. (Citation2007) further considered the potential of soluble and insoluble fiber in reducing the risk of these cancers. For soluble fiber, mean intakes of 2.6 g/day compared to 1.2 g/day significant reduced colorectal and colon cancer risk by 33% (p for trend = 0.022) and 45% (p for trend = 0.002), respectively. Again, no significant reduction in risk was seen for rectal cancer. The same was observed for insoluble fiber, where mean intakes of 9.6 g/day compared to 5.3 g/day reduced the risk of colorectal and colon cancer by 23% (p for trend = 0.041) and 37% (p for trend = 0.004), respectively, but not the risk of rectal cancer. However, determining the independent effects of soluble and insoluble fiber on cancer prevention is extremely difficult as they are typically consumed together and co-exist within the same foods (Dhingra et al. Citation2012; Khanum et al. Citation2000; Spiller, Amen, and Kritchevsky Citation1975). The lack of effect of fiber on rectal cancer may be due to low residence time in that part of the digestive system (McNeil and Rampton Citation1981).
Dietary fiber intake has also been shown to have a preventative role in breast cancer, although the effects are weaker compared to colorectal cancer (Aune et al. Citation2012). A study by Cade, Burley, and Greenwood (Citation2007) of the UK Women’s Cohort Study (UKWCS) involved 15,951 pre-menopausal women and 17,781 post-menopausal women, aged between 35- and 69-years-old, at baseline (Cade, Burley, and Greenwood Citation2007). As in the previous studies, FFQs were used to obtain each participant’s dietary data and to calculate dietary fiber intakes. A total dietary fiber intake of at least 30 g/day compared to less than 20 g/day was seen to reduce the risk of pre-menopausal breast cancer significantly by 52% (p for trend = 0.01) but not post-menopausal breast cancer. Foods containing dietary fiber, such as cereal products and vegetables, generally contain other substances such as isoflavones and lignans (Adlercreut et al. Citation2000). These can be broken down into molecules know as phytestrogens. Phytestrogens have similar structures to estrogen and may compete for estrogen receptor binding sites, stopping estrogen from binding and so preventing the estrogenic pathways which can lead to pre-menopausal breast cancer (Stoll Citation1996). A study by Li et al. (Citation2013) showed a significantly protective effect of fiber against breast cancer in pre-menopausal women (OR = 0.38, p for trend = 0.08) and the effect was more pronounced for prevention of estrogen receptor (ER) negative tumors. However, the effect was not observed for post-menopausal women, irrespective of ER tumor type (Li et al. Citation2013).
Overall, there is strong and consistent evidence provided by epidemiological trials supporting a preventative role of dietary fiber in obesity and its related diseases ().
Table 1. Reductions in the risks of the various obesity-related diseases, when comparing higher and lower total dietary fiber intakes in epidemiological studies.
It is important to acknowledge that epidemiological study designs can prone to bias, for example in participant selection by country or dietary pattern (The BMJ, XXXX). Therefore, caution must be exerted to avoid generalization to the global population. In particular, quite a lot of epidemiological studies focus on populations that consume high amounts of cereal wholegrains, but low amounts of fruits, vegetables, nuts and legumes. More fiber interventions are needed in a wider range of dietary contexts (Das et al. Citation2021).
Potential mechanisms by which fiber prevents obesity and associated diseases
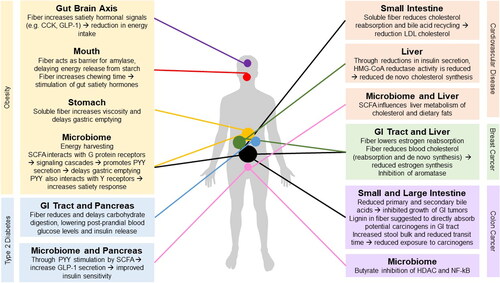
As suggested by the epidemiological evidence, dietary fiber may have protective effects against obesity and its related diseases (T2DM, CVD, colon and breast cancer). Several mechanisms have been proposed which include direct effects of fiber on gut physiology with consequences on digestion, absorption and appetite; as well as indirect effects through the microbiome ().
Direct effects of fiber on gut physiology and appetite
Obesity prevention
Lower energy intakes may help individuals achieve and regulate energy balance for weight maintenance (NICE. Citation2015; Church and Martin Citation2018). Heaton (Citation1973) proposed three main mechanisms in which dietary fiber may reduce energy intake. The first mechanism involves the replacement of macronutrients consumed by dietary fiber. Fiber reduces energy density of foods, and therefore high-fiber diets will have lower energy content compared for the same portion size.
Secondly, foods containing fiber typically need more chewing before they can be ingested. This requirement for chewing not only slows down the rate of food consumption but also initiates feelings of satiety by stimulating the release of gut hormones, such as cholecystokinin (CCK) and glucagon-like-peptide-1 (GLP-1) (Li et al. Citation2011; Haber et al. Citation1977; Miquel-Kergoat et al. Citation2015; Hogenkamp and Schiöth Citation2013; Austin and Marks Citation2009; Slavin and Green Citation2007). As fiber increases feelings of fullness and lowers the number of calories ingested, an overall reduction in energy intake results which prevents obesity development (Astrup Citation2005). Moreover, soluble dietary fiber, has been specifically linked to increased feelings of satiety and a reduced overall energy intake (Adam et al. Citation2015; Pasman et al. Citation1997). Soluble fiber is thought to increase satiety by increasing viscosity of intestinal contents, producing gel-like structures, and its ability to undergo fermentation in the small intestine (Fiszman and Varela Citation2013).
The final mechanism suggested by Heaton (Citation1973) is that fiber reduces the energy availability from food. For example, when comparing wholegrain products to their refined alternatives, fiber-rich cell walls act as barriers to digestive enzymes (Edwards et al. Citation2021; Holland et al. Citation2020). This “barrier” reduces the bioavailability of energy-yielding components in the GI tract (Edwards Citation1990). Soluble fibers increase the viscosity of the GI lumen content (Slavin and Green Citation2007; Dikeman and Fahey Citation2006), affecting digestive enzyme activity but also slowing down the rate of gastric emptying. Together, soluble fiber can increase the duration of which satiety signals are released (Fiszman and Varela Citation2013). Fiber also extends oral exposure to food and generates more satiating textures (Wanders et al. Citation2013; Morell et al. Citation2014).
T2dm prevention
With regards to T2DM, increased viscosity has been shown to slow down the rate at which carbohydrates are digested and absorbed in the small intestine (Jenkins Citation1979; Topping et al. Citation1988). Fiber forms a viscous gel acting as a diffusion barrier between the epithelial cells and available glucose (Fabek et al. Citation2014; Flourie et al. Citation1984). Fiber components may also inhibit α-amylase activity (Ou et al. Citation2001). Altogether, this reduces the rate at which glucose is absorbed into the blood stream, and so lowers postprandial blood glucose levels (Yu, et al. Citation2014). As blood glucose levels are reduced, so is the release of insulin (Ludwig Citation1999). This may generate an overall increase in the insulin sensitivity of tissues through increased activity of insulin receptors, their subsequent tyrosine kinase pathways, and further signaling molecules (Pirola, Johnston, and Van Obberghen Citation2004; Song et al. Citation2000; Robertson et al. Citation2003).
CVD Prevention
Reductions in postprandial blood glucose levels by fiber have also been linked to CVD prevention (Cameron-Smith, Collier, and O’Dea Citation1994; Gunness and Gidley Citation2010). Insulin is known to promote the action of the hepatic enzyme 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase (Gunness and Gidley Citation2010; Harris et al. Citation2000; Istvan and Deisenhofer Citation2001). HMG-CoA reductase is involved in cholesterol synthesis as it increases the rate at which cholesterol is produced within the liver. It does this by acting as a catalyst for the rate limiting step of cholesterol’s production (Rodwell Citation1976). This step involves the conversion of HMG-CoA to mevalonate (Espenshade and Hughes Citation2007), which undergoes further modifications producing squalene and lanosterol, before finally ending up as cholesterol (Röhrl and Stangl Citation2018). Therefore, inhibition of HMG-CoA reductase may result in the prevention of excess cholesterol being synthesized and released into circulation by the liver, and so may reduce the risk of atherosclerosis and so CVD (Breuer Citation2005; Rafieian-Kopaei et al. Citation2014).
The second proposed mechanism is through the inhibition of the re-absorption and recycling of bile acids (Kritchevsky Citation1987; Eastwood Citation2019; Story and Lord Citation1987). Some studies suggest that fiber binds to bile acids (Naumann et al. Citation2019), although the physico-chemical mechanisms behind this behavior are not clear.
Colorectal and breast cancer prevention
The interaction between fiber and bile may also have a role in colorectal cancer prevention. Primary and secondary bile acids, at high concentrations, have been suggested as potential carcinogens that promote the growth of tumors within the GI tract (Bernstein et al. Citation2005). Secondary bile acids in particular have been linked to the promotion of tumor growth (Nagengast et al. Citation1995) through damage to epithelial cells lining the colon, which in turn may increase the intestinal wall’s susceptibility to carcinogens (Ajouz, Mukherji, and Shamseddine Citation2014). Additionally, secondary bile acids promote the production of reactive oxygen species (ROS), which have roles in cell proliferation and resistance to cellular apoptosis (Nguyen et al. Citation2018; Waris and Ahsan Citation2006).
Lignin, a phenolic-type of dietary fiber, has also been shown to protect against colorectal cancer development by directly adsorbing potential carcinogens within the GI tract (Ferguson and Harris Citation1996).
Another mechanism by which fiber may prevent colorectal cancer is through its ability to increase stool bulk (Harris and Ferguson Citation1993), which is seen particularly with insoluble fiber (Sloan and McRorie Citation2017). Increasing stool bulk can dilute potential carcinogens within the GI tract and decrease transit time (Yoon and Kim Citation2018). A dilution of carcinogens would reduce the likelihood of them coming into contact with the gut mucosa and epithelial cells, therefore reducing the chance of carcinogens being absorbed and or causing damage to the cells, resulting in a reduced risk of tumor formation (Masrul and Nindrea Citation2019). Additionally, decreased transit time reduces the probability of carcinogens coming into contact with epithelial cells (Cameron, Hardman, and Heitman Citation1997).
Lastly, proposed mechanisms for the prevention of breast cancer by dietary fiber involve estrogen metabolism (Rose Citation1992). These estrogen-related pathways are major contributors of breast cancer development (Key et al. Citation2003). Estrogen-related mechanisms involve the inhibition of estrogen re-absorption within the intestines (Adlercreutz et al. Citation1987; Lattimer and Haub Citation2010), as seen with bile, cholesterol, and carcinogens, and the inhibition of estrogen synthesis (Zhang et al. Citation2011). These mechanisms reduce estrogen levels (Rock et al. Citation2004; Gaskins et al. Citation2009), of which high levels have been linked to the development of tumors within breast tissue (Travis and Key Citation2003). Additionally, estrogen and their receptors are known to promote the growth and development of these breast tumors (Russo and Russo Citation2006; Sui, Zhang, and Fan Citation2011), with estrogen receptors regulating cell proliferation (Lee et al. Citation2012). Furthermore, the most common form of breast cancer is one that is estrogen receptor positive (Turner et al. Citation2017), further demonstrating evidence for estrogen’s role in breast cancer. Therefore, if fiber can increase excretion of estrogen via decreased absorption (Upson et al. Citation2013), binding of estrogen to its receptors would be reduced, preventing the initiation of breast tissue cell growth, overall potentially reducing the risk of breast tumor development.
Finally, estrogen synthesis may be reduced by inhibition of the aromatase enzyme (Adlercreutz et al. Citation1993), which produces the estrogens estrone (E1) and estradiol (E2) from androstenedione and testosterone, respectively (Thomas and Potter Citation2013). Estrogen synthesis may also be reduced by the reductions in cholesterol levels, as previously mentioned, as estrogen can also be produced via de novo pathways from cholesterol (van Duursen Citation2017). Overall, these two pathways would further decrease circulating estrogen levels, potentially preventing the development of breast cancer.
Fiber and obesity/disease prevention through Microbiome-Related mechanisms
Dietary fiber has been shown to reduce the risk of obesity and its related diseases through indirect mechanisms mediated by the microbiome (Cronin et al. Citation2021). The term “microbiome” is defined as “a community of microorganisms (such as bacteria, fungi and viruses) that inhabit a particular environment and especially the collection of microorganisms living in or on the human body” (Merriam-Webster Citation2020a). The term “microbiota” is defined as “the microscopic organisms of a particular environment” (Merriam-Webster Citation2020b), and is a term often used interchangeably with “microbiome” when referring to the bacteria existing within an individual’s GI tract.
Fiber can alter gut microbial populations (David et al. Citation2014). Anaerobic fermentation of dietary fibers within the GI tract produce SCFAs amongst other products (Andoh, Tsujikawa, and Fujiyama Citation2003; Ríos-Covián et al. Citation2016; Wong et al. Citation2006). The interaction between fiber and microbes is complex, and likely to involve a whole range of yet undiscovered microbes, enzymes and metabolites (Cronin et al. Citation2021; Klassen et al. Citation2021). There are three main SCFAs thought to be produced by these gut bacteria; acetate, propionate and butyrate (Topping Citation1996). Butyrate is thought to be the major SCFA produced, providing energy to the colonocytes (Hamer et al. Citation2008). However, all SCFAs produced can be utilized by enterocytes in the human colon, and/or can be transported into the bloodstream via movement across the GI tract epithelium (Tan et al. Citation2014). While a lot of research has emphasized the roles of SCFA in obesity and disease prevention (Tan et al. Citation2014; Mandaliya, Patel, and Seshadri Citation2018), the microbiome may have other protective effects by altering the absorption of nutrients and modulation of low-grade inflammatory pathways (Baothman et al. Citation2016).
Obesity prevention through Microbiome-Related mechanisms
Studies have demonstrated that dietary fiber can influence obesity development through indirect mechanisms involving the microbiome. Firstly, with regards to energy intake, there has been some suggestion that obese individuals contain a microbiome made up of bacteria that are highly efficient at extracting energy from the food components entering the GI tract compared to lean individuals (Turnbaugh et al. Citation2006). This energy extraction process is often referred to as “energy harvesting” (Flint Citation2011). If that energy taken in is not expended, this would lead to weight gain and eventually could develop into obesity (Hill Citation2006). Evidence for this mechanism is provided by Fernandes et al. (Citation2014) who observed that obese individuals showed significantly higher numbers of total SCFAs within their GI tract when compared to lean individuals (p = 0.02). This may indicate that more food material is being utilized by the gut bacteria and so may provide more energy to the host.
Studies have also demonstrated that obese individuals are seen to have increased populations of Firmicutes along with decreases in populations of Bacteroidetes (Ley et al. Citation2006). These changes in bacterial populations have been linked to an enhanced absorption of energy (Turnbaugh et al. Citation2006). However, contradictory results for this proposal were observed by both Fernandes et al. (Citation2014) and Mariat et al. (Citation2009), whereby no significant differences in Bacteroidetes and Firmicutes were seen between obese and lean individuals (Fernandes et al. Citation2014), and that moreover the ratio between these two phyla change with age (Mariat et al. Citation2009). This suggests that these phyla ratios are not characteristics of an obese microbiome. Additionally, Schwiertz et al. (Citation2010) saw a significant increase in Bacteroidetes among individuals who were overweight or obese.
Contradictory evidence for these changes seen within obese individuals compared to lean individuals is further provided by the SCFAs produced via fermentation of dietary fiber. Butyrate has been shown to increase energy expenditure and is said to be protective against obesity caused by dietary factors (Gao et al. Citation2009). As Firmicutes are thought to predominantly produce the butyrate and Bacteroidetes are thought to produce acetate and propionate (Macfarlane and Macfarlane Citation2003), then increases in Firmicutes should help prevent obesity. However, Chakraborti (Citation2015) proposed that butyrate and propionate both exerted anti-obesogenic properties, and so increases in Bacteroidetes may help prevent obesity. Furthermore, Chakraborti (Citation2015) also postulated that acetate could promote obesity through its ability to initiate lipogenesis in the liver, suggesting that decreases in Bacteroidetes would prevent obesity. This further contradicts the initial findings of Ley et al. (Citation2006). This lack of coherence warrants the need for further studies regarding specific gut bacteria and SCFA, and their roles in obesity. Nevertheless, other studies have been able to demonstrate other potential mechanistic pathways in which dietary fiber may indirectly prevent obesity through its microbiome-related effects.
One such final mechanism involves two G-protein-coupled receptors, GPR41 and GPR43. GPR41 and GPR43 are also referred to as FFAR3 (free fatty acid receptor 3) and FFAR2 (free fatty acid receptor 2), respectively (Wang et al. Citation2009). Both GPR41 and GPR43 exist on enteroendocrine cells known as L-cells in epithelial cells within the colon, in adipocytes, and in blood mononuclear cells (Ang and Ding Citation2016; Karaki et al. Citation2008; Kimura et al. Citation2014). SCFAs are able to bind to these receptors and generate signaling cascades involved in metabolic regulation (Ulven Citation2012). One of these cascades involves the secretion of peptide YY (PYY), which is a gut hormone (Bohórquez et al. Citation2011). PYY is is considered to be an anorectic gut hormone as it reduces appetite by delaying gastric emptying (Garattini et al. Citation1979; De Silva and Bloom Citation2012).
Evidence for the involvement of PYY was provided by studies that identified low levels of PYY in obese individuals (Viardot et al. Citation2008; Gueugnon et al. Citation2012; Brownley et al. Citation2010; le Roux et al. Citation2006). Batterham et al. (Citation2003) demonstrated significantly low levels of PYY after fasting and after the consumption of a meal in obese individuals when compared with lean individuals (p < 0.001). Therefore, dietary fiber intake would enable greater amounts of SCFAs to be produced, activating GPR41 and GPR43, stimulating the release of PYY by the L-cells, and ultimately suppressing appetite, helping to prevent weight gain.
PYY is thought to provide these anti-obesogenic effects through binding with neuropeptide Y receptors (Y1, Y2, Y4, Y5) (Yulyaningsih et al. Citation2011), specifically the Y2 receptor. Activations of the Y receptors prevent certain pathways, for example the degradation of cyclic AMP (cAMP) (Larhammar Citation1996). cAMP is a very important molecule involved in metabolism as it can promote glycogenolysis and lipolysis within muscles and adipose tissue, respectively (Sutherland and Robison Citation1969). Therefore, encouraging the body to use up stored energy, which may help to prevent obesity.
Additionally, the Y2 receptor, along with other Y receptors, are present within areas of the brain, such as the hypothalamus which also have crucial functions in regulating metabolism (Fetissov, Kopp, and Hökfelt Citation2004). The hypothalamus helps to control appetite by receiving and relaying central and peripheral pathway signals (Suzuki et al. Citation2010). One peripheral pathway is the binding of PYY to Y2 receptors located on vagal afferent nerve terminals (Ueno et al. Citation2008). Here, pro-opiomelanocortin neurons located within the arcuate nucleus get activated, stimulating these satiety signals and generating decreases in appetite (Heisler and Lam Citation2017). Thus, aiding in the prevention of weight gain and so possibly obesity.
Multiple studies have suggested gender specific microbiome dysbiosis with effects on obesity and health outcomes (Kim et al. Citation2020{Haro, 2016 #327]}.
T2dm prevention through Microbiome-Related mechanisms
Both GPR41 and GPR43 are thought to have a role in T2DM as well as obesity. Both GPR41 and GPR43 have demonstrated roles in the regulation of glucose homeostasis and pancreatic ß-cell insulin release (Swaminath Citation2008), on binding with the SCFAs (Ulven Citation2012). This binding generates signaling cascades involving not only the release of PYY, as seen in the previous obesity section, but also the release of GLP-1 by enteroendocrine L-cells (Cani, Everard, and Duparc Citation2013). These molecules may also exert anti-diabetic properties (Li et al. Citation2017), as well as the anti-obesogenic previously mentioned.
GLP-1 stimulates the release of insulin by the pancreatic ß-cells and inhibits the secretion of glucagon (Zhang et al. Citation2017; Müller et al. Citation2019). This enables rising blood sugar levels to be lowered back to normal baseline levels (Meier Citation2012). Therefore, an increased dietary fiber intake generates an increase in beneficial bacteria, leading to an increase in SCFA production and ultimately enhanced activation of GPR43. This enables improved pancreatic ß-cell activity and greater insulin production, generating an overall improvement in blood glucose homeostasis within individuals with T2DM.
Evidence for the activation of GPR41 and GPR43 by SCFAs, specifically acetate, propionate, and butyrate, was provided by Brown et al. (2002) and by Le Poul et al. (Citation2003). Le Poul et al. (Citation2003) further demonstrated that each of these SCFAs exerted different levels of efficacy for each receptor. It was seen that propionic acid was the most effective SCFA at activating both GPR41 and GPR43. Butyrate followed propionate in effectively activating GPR41 and was more effective when compared to acetate. Acetate then followed propionate in effectively activating GPR43 and was more effective when compared to butyrate (Le Poul et al. Citation2003).
This evidence has resulted in the consideration for GPR41 and GPR43 as possible targets for drug therapies in T2DM and obesity (Ang and Ding Citation2016; Rayasam et al. Citation2007; Ichimura et al. Citation2014).
CVD Prevention through Microbiome-Related mechanisms
The SCFA propionate is thought to exert a protective effect against CVD. Propionate appears to play a role in the regulation of cholesterol production in the liver (Ooi and Liong Citation2010). Wright, Anderson, and Bridges (Citation1990) also reported a possible role in preventing the synthesis of fatty acids. These effects were also observed by Chen, Anderson, and Jennings (Citation1984) in an animal study whereby the rats fed a propionate enriched cholesterol diet had significantly lower cholesterol levels within their blood and liver compared to those fed diets without propionate. The mechanism by which propionate is thought to achieve this is through its ability to block the utilization of acetate for the synthesis of cholesterol (Wolever, Spadafora, and Eshuis Citation1991). Acetate is used to make acetyl-coenzyme A (Acetyl-CoA) in the liver, which is a precursor for cholesterol biosynthesis, involving HMG-CoA reductase as a rate limiting step (Park et al. Citation2018). However, a later study conducted by Wolever et al. (Citation1995) saw no significant effect of propionate on cholesterol levels but did observe some evidence to suggest its role in the prevention of acetate utilization in lipid synthesis. Furthermore, the mechanisms by which propionate is able to prevent cholesterol and lipid synthesis remain unclear, with other studies showing conflicting evidence against it. For example, Hara et al. (Citation1998) found that it may actually be the SCFA acetate, also produced during the anaerobic fermentation of dietary fibers, that has the ability to reduce blood cholesterol levels rather than propionate.
Colorectal cancer prevention through Microbiome-Related mechanisms
A role for SCFA butyrate in the prevention of colorectal cancer has been proposed, mainly through its ability to induce apoptosis and prevent proliferation of cancer cells (Clausen, Bonnén, and Mortensen Citation1991). However, there is thought to be a paradox called the “butyrate paradox” as butyrate is seen to cause proliferation among normal healthy colonic cells whilst at the same time preventing growth and proliferation of colonic cancer cells (Scheppach, Bartram, and Richter Citation1995). In terms of colon cancer prevention, butyrate is thought to achieve this through effects on the immune system pathways, whereas its role in bowel cancer prevention is thought to be through inducing apoptosis within cancer cells by preventing the activity of histone deacetylase (HDAC) enzymes (Chen and Vitetta Citation2018).
With reference to butyrate’s effects on the immune system for preventing colon cancer, this mechanism is believed to involve the butyrate G-protein-coupled receptor GPR109A (Singh et al. Citation2014). In colon cancer, this receptor is essentially switched off but on binding with butyrate is able to be switched back on. This activation on binding with butyrate enables apoptosis to occur within colon cancer cells through modulation of for example Bcl2. The action of nuclear factor kB (NFkB) is also prevented by the binding of butyrate with GPR109A. However, this occurs in both the colon cancer cells and the normal healthy colonic cells (Thangaraju et al. Citation2009). NFkB is an important molecule within the regulation of immune responses and inflammation as it is as a transcription factor (Dolcet et al. Citation2005). NFkB is also specifically associated with proinflammatory cytokines, thus promoting inflammation (Schottelius and Baldwin Citation1999). Therefore, if its activity is prevented by butyrate and GPR109A binding, and GPR109A being thought to act as an anti-inflammatory itself, then protection against both overall colon cancer development and colon cancer initiated by inflammation is enhanced (Elangovan et al. Citation2014).
A second mechanism involving butyrate in cancer prevention is via the inhibition of HDAC enzymes. This preventative effect is thought to be due to HDAC inhibition generating the promotion of the tumor suppressor gene p21, which enables even greater inhibition of cyclin-D kinases (CDK) (Hassig, Tong, and Schreiber Citation1997). As CDK4 and CDK6 are known to encourage the proliferation and growth of cells through the advancement of the cell cycle, their inhibition is important in preventing the growth and development of cancerous cells and stopping their progression to tumors (Musgrove et al. Citation2011).
Lastly, this inactivation of HDAC enzymes by butyrate has also been seen to prevent inflammation and growth of colon cancer cells by altering and preventing the pathways involving the activation of the transcription factor NFkB within these cancer cells (Place, Noonan, and Giardina Citation2005). This is thought to be the case as NFkB aids in the formation, growth, and survival of tumors via the promotion of cell proliferation and resistance to apoptosis (Park and Hong Citation2016; Karin et al. Citation2002; Nakajima and Kitamura Citation2013), as previously mentioned. Therefore, both HDAC and NFkB inhibition by the presence of butyrate, as a result of dietary fiber consumption, would help to prevent the development and progression of colorectal cancer.
General conclusions
Overall, this review has found evidence from both epidemiologically and mechanistic studies, that dietary fiber intake may protect against obesity and its related chronic diseases.
A harmonized international definition of dietary fiber, together with improved tools for fiber intake assessement, are needed to consolidate dietary fiber’s role in disease prevention. This would ensure that studies conducted globally, whether epidemiological or interventional, would be expressing fiber and its components, in a comparable way. This in turn would generate a robust body of evidence to justify, ascertain, and strengthen the associations between dietary fiber intakes and disease prevention.
Furthermore, dietary intervention trials in a wide range of dietary contexts are required to reduce selection bias. Dietary trials with clearly defined high-fiber diets, or well characterized dietary fiber supplements are also needed. This enable the elucidation of effects of specific dietary fiber components to be understood.
Finally, although this review addresses obesity and its related diseases separately, the mechanisms clearly demonstrate their disease pathways are somewhat interlinked. The same enzymes, hormones, receptors and or pathways are often affected, and one disease and/or risk factor often leads to the development of another, as is often seen in people with metabolic syndrome. Individual responses to diets, for example by gender and ethnicity, as well as the interaction of fiber with other dietary components, need to be further elucidated. It is worth noting that fiber-rich foods are also often rich in bioactive phytochemicals. These compounds may also show protective effects against obesity and associated diseases (Diotallevi et al. Citation2020; Bordoni et al. Citation2019).
To conclude, there is strong evidence to suggest a role for dietary fiber in the prevention of obesity and its related diseases, with further research required to substantiate the evidence in a range of dietary contexts and to fully understand the mechanisms involved. This could then inform nutrient and food-based dietary guidelines for consumers, as well as guide industrial formulations of higher fiber products.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Adam, C. L., P. A. Williams, K. E. Garden, L. M. Thomson, and A. W. Ross. 2015. Dose-dependent effects of a soluble dietary fibre (pectin) on food intake, adiposity, gut hypertrophy and gut satiety hormone secretion in rats. PloS One 10 (1):e0115438. doi: 10.1371/journal.pone.0115438.
- Adlercreut, H., W. Mazur, K. Stumpf, A. Kilkkinen, P. Pietinen, K. Hultén, and G. Hallmans. 2000. Food containing phytoestrogens, and breast cancer. BioFactors (Oxford, England) 12 (1–4):89–94. doi: 10.1002/biof.5520120114.
- Adlercreutz, H., C. Bannwart, K. Wähälä, T. Mäkelä, G. Brunow, T. Hase, P. J. Arosemena, J. T. Kellis, and L. E. Vickery. 1993. Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens. The Journal of Steroid Biochemistry and Molecular Biology 44 (2):147–53. doi: 10.1016/0960-0760(93)90022-o.
- Adlercreutz, H., E. Hämäläinen, S. L. Gorbach, B. R. Goldin, M. N. Woods, L. Swenson Brunson, and J. T. Dwyer. 1987. Association of diet and sex hormones in relation to breast cancer. European Journal of Cancer and Clinical Oncology 23 (11):1725–6. doi: 10.1016/0277-5379(87)90459-7.
- Ajouz, H., D. Mukherji, and A. Shamseddine. 2014. Secondary bile acids: An underrecognized cause of colon cancer. World Journal of Surgical Oncology 12 (1):164. doi: 10.1186/1477-7819-12-164.
- Andoh, A., T. Tsujikawa, and Y. Fujiyama. 2003. Role of dietary fiber and short-chain fatty acids in the colon. Current Pharmaceutical Design 9 (4):347–58. doi: 10.2174/1381612033391973.
- Ang, Z., and J. L. Ding. 2016. GPR41 and GPR43 in obesity and inflammation - Protective or causative? Frontiers in Immunology 7:28. doi: 10.3389/fimmu.2016.00028.
- Astrup, A. 2005. The satiating power of protein-a key to obesity prevention? The American Journal of Clinical Nutrition 82 (1):1–2. doi: 10.1093/ajcn.82.1.1.
- Aune, D., D. S. M. Chan, D. C. Greenwood, A. R. Vieira, D. A. N. Rosenblatt, R. Vieira, and T. Norat. 2012. Dietary fiber and breast cancer risk: A systematic review and meta-analysis of prospective studies. Annals of Oncology: Official Journal of the European Society for Medical Oncology 23 (6):1394–402. doi: 10.1093/annonc/mdr589.
- Aune, D., D. S. M. Chan, R. Lau, R. Vieira, D. C. Greenwood, E. Kampman, and T. Norat. 2011. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ (Clinical Research ed.) 343:d6617. doi: 10.1136/bmj.d6617.
- Austin, J., and D. Marks. 2009. Hormonal regulators of appetite. International Journal of Pediatric Endocrinology 2009 (1):141753–9. doi: 10.1155/2009/141753.
- Australian Government National Health and Medical Research Council. 2019. Dietary fibre. https://www.nrv.gov.au/nutrients/dietary-fibre.
- Baothman, O. A., M. A. Zamzami, I. Taher, J. Abubaker, and M. Abu-Farha. 2016. The role of gut microbiota in the development of obesity and diabetes. Lipids in Health and Disease 15 (1):108. doi: 10.1186/s12944-016-0278-4.
- Batterham, R. L., M. A. Cohen, S. M. Ellis, C. W. Le Roux, D. J. Withers, G. S. Frost, M. A. Ghatei, and S. R. Bloom. 2003. Inhibition of food intake in obese subjects by peptide YY3–36. New England Journal of Medicine 349 (10):941–8. doi: 10.1056/NEJMoa030204.
- Bazzano, L. A., J. He, L. G. Ogden, C. M. Loria, P. K. Whelton, and National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. 2003. Dietary fiber intake and reduced risk of coronary heart disease in US men and women: The National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Archives of Internal Medicine 163 (16):1897–904. doi: 10.1001/archinte.163.16.1897.
- Bernstein, H., C. Bernstein, C. M. Payne, K. Dvorakova, and H. Garewal. 2005. Bile acids as carcinogens in human gastrointestinal cancers. Mutation Research 589 (1):47–65. doi: 10.1016/j.mrrev.2004.08.001.
- Beuther, D. A., and E. R. Sutherland. 2007. Overweight, obesity, and incident asthma. American Journal of Respiratory and Critical Care Medicine 175 (7):661–6. doi: 10.1164/rccm.200611-1717OC.
- Birchenough, G., B. O. Schroeder, F. Bäckhed, and G. C. Hansson. 2019. Dietary destabilisation of the balance between the microbiota and the colonic mucus barrier. Gut Microbes 10 (2):246–50. doi: 10.1080/19490976.2018.1513765.
- Bohórquez, D. V., R. Chandra, L. A. Samsa, S. R. Vigna, and R. A. Liddle. 2011. Characterization of basal pseudopod-like processes in ileal and colonic PYY cells. Journal of Molecular Histology 42 (1):3–13. doi: 10.1007/s10735-010-9302-6.
- Bordoni, A., C. Boesch, C. Malpuech-Brugère, C. Orfila, and L. Tomás-Cobos. 2019. The role of bioactives in energy metabolism and metabolic syndrome. The Proceedings of the Nutrition Society 78 (3):340–50. doi: 10.1017/S0029665119000545.
- Breuer, H. 2005. Low density lipoprotein cholesterol and coronary heart disease—Lower is better. European Cardiology Review 1 (1):1–6.
- British Nutrition Foundation. 2018. Dietary fibre. https://www.nutrition.org.uk/healthyliving/basics/fibre.html?limit=1&__cf_chl_jschl_tk__=d8836767544fe83291afb4f2aaed67f1625eff94-1602150406-0-Ad1dk1qy68A8MFauu7PxpVL_n7Kl2ucf-cGXtTigN0OvDSSgqp6HpYAmdE7pVSrXS47wH_GRVqroNQ2h_-N4U1l SlojmYjiHgBoY_PENYwIEETdiMgiHNRWR0ygJxIyWGO1lLm iG5VAD6DAfYdMymQfQGBxIXwR9J6o12s1aTQUEjwBf3km32 9YyCRqlH59p_dlIjtAsZFfNVgFR-EmVxQRijsz-dNNEjSOY ghj_i0bfxecGIt_Mmern6j5ZuyW1RMcioszuKOGy-5TRwtT M6_VN10DAnOwRSNslkn1svX2-jpes1P9YeQn4AIeaPXOkST keOK73o2ER957IfZnk2EHnIJopSfllf0zzspxSnbSyhORn7 xhDUHSFmDV_H-s02w.
- British Nutrition Foundation. 2018. Dietary fibre. https://www.nutrition.org.uk/nutritionscience/nutrients-food-and-ingredients/dietary-fibre.Q.
- Brown, A. J., S. M. Goldsworthy, A. A. Barnes, M. M. Eilert, L. Tcheang, D. Daniels, A. I. Muir, M. J. Wigglesworth, I. Kinghorn, N. J. Fraser, et al. 2003. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. The Journal of Biological Chemistry 278 (13):11312–9. doi: 10.1074/jbc.M211609200.
- Brownley, K. A., S. Heymen, A. L. Hinderliter, and B. MacIntosh. 2010. Effect of glycemic load on peptide-YY levels in a biracial sample of obese and normal weight women. Obesity 18 (7):1297–303. doi: 10.1038/oby.2009.368.
- Cade, J. E., V. J. Burley, D. C. Greenwood, and The UK Women’s Cohort Study Steering Group. 2007. Dietary fibre and risk of breast cancer in the UK Women’s Cohort Study. International Journal of Epidemiology 36 (2):431–8. doi: 10.1093/ije/dyl295.
- Cameron, I. L., W. E. Hardman, and D. W. Heitman. 1997. The nonfermentable dietary fiber lignin alters putative colon cancer risk factors but does not protect against DMH-induced colon cancer in rats. Nutrition and Cancer 28 (2):170–6. doi: 10.1080/01635589709514571.
- Cameron-Smith, D., G. R. Collier, and K. O’Dea. 1994. Effect of soluble dietary fibre on the viscosity of gastrointestinal contents and the acute glycaemic response in the rat. The British Journal of Nutrition 71 (4):563–71. doi: 10.1079/bjn19940163.
- Cani, P. D., A. Everard, and T. Duparc. 2013. Gut microbiota, enteroendocrine functions and metabolism. Current Opinion in Pharmacology 13 (6):935–40. doi: 10.1016/j.coph.2013.09.008.
- Chakraborti, C. K. 2015. New-found link between microbiota and obesity. World Journal of Gastrointestinal Pathophysiology 6 (4):110–9. doi: 10.4291/wjgp.v6.i4.110.
- Chan, Y.-M., S. Aufreiter, S. J. O’Keefe, and D. L. O’Connor. 2019. Switching to a fibre-rich and low-fat diet increases colonic folate contents among African Americans. Applied Physiology, Nutrition, and Metabolism = Physiologie Appliquee, Nutrition et Metabolisme 44 (2):127–32.
- Chen, J., and L. Vitetta. 2018. Inflammation-modulating effect of butyrate in the prevention of colon cancer by dietary fiber. Clinical Colorectal Cancer 17 (3):e541–e544. doi: 10.1016/j.clcc.2018.05.001.
- Chen, J.-P., G.-C. Chen, X.-P. Wang, L. Qin, and Y. Bai. 2017. Dietary fiber and metabolic syndrome: A meta-analysis and review of related mechanisms. Nutrients 10 (1):24. doi: 10.3390/nu10010024.
- Chen, W. J., J. W. Anderson, and D. Jennings. 1984. Propionate may mediate the hypocholesterolemic effects of certain soluble plant fibers in cholesterol-fed rats. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, NY) 175 (2):215–8. doi: 10.3181/00379727-175-41791.
- Chooi, Y. C., C. Ding, and F. Magkos. 2019. The epidemiology of obesity. Metabolism: Clinical and Experimental 92:6–10. doi: 10.1016/j.metabol.2018.09.005.
- Church, T., and C. K. Martin. 2018. The obesity epidemic: A consequence of reduced energy expenditure and the uncoupling of energy intake? Obesity (Silver Spring, MD) 26 (1):14–6. doi: 10.1002/oby.22072.
- Clausen, M. R., H. Bonnén, and P. B. Mortensen. 1991. Colonic fermentation of dietary fibre to short chain fatty acids in patients with adenomatous polyps and colonic cancer. Gut 32 (8):923–8. doi: 10.1136/gut.32.8.923.
- Cronin, P., S. A. Joyce, P. W. O’Toole, and E. M. O’Connor. 2021. Dietary fibre modulates the gut microbiota. Nutrients 13 (5):1655. doi: 10.3390/nu13051655.
- Dahm, C. C., R. H. Keogh, E. A. Spencer, D. C. Greenwood, T. J. Key, I. S. Fentiman, M. J. Shipley, E. J. Brunner, J. E. Cade, V. J. Burley, et al. 2010. Dietary fiber and colorectal cancer risk: A nested case-control study using food diaries. Journal of the National Cancer Institute 102 (9):614–26. doi: 10.1093/jnci/djq092.
- Das, M., P. Das, L. Chatterjee, and A. Gohain. 2021. Impact of fibre supplement on the obesity related risk factors. Pharma Innovation 10 (3):412–6.
- David, L. A., C. F. Maurice, R. N. Carmody, D. B. Gootenberg, J. E. Button, B. E. Wolfe, A. V. Ling, A. S. Devlin, Y. Varma, M. A. Fischbach, et al. 2014. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505 (7484):559–63.
- De Silva, A., and S. R. Bloom. 2012. Gut hormones and appetite control: A focus on PYY and GLP-1 as therapeutic targets in obesity. Gut and Liver 6 (1):10–20. doi: 10.5009/gnl.2012.6.1.10.
- Dhingra, D., M. Michael, H. Rajput, and R. T. Patil. 2012. Dietary fibre in foods: A review. Journal of Food Science and Technology 49 (3):255–66. doi: 10.1007/s13197-011-0365-5.
- Dikeman, C. L., and G. C. Fahey. 2006. Viscosity as related to dietary fiber: A review. Critical Reviews in Food Science and Nutrition 46 (8):649–63. doi: 10.1080/10408390500511862.
- Diotallevi, C., F. Fava, M. Gobbetti, and K. Tuohy. 2020. Healthy dietary patterns to reduce obesity-related metabolic disease: Polyphenol-microbiome interactions unifying health effects across geography. Current Opinion in Clinical Nutrition & Metabolic Care 23 (6):437–44. doi: 10.1097/MCO.0000000000000697.
- Dolcet, X., D. Llobet, J. Pallares, and X. Matias-Guiu. 2005. NF-kB in development and progression of human cancer. Virchows Archiv: An International Journal of Pathology 446 (5):475–82. doi: 10.1007/s00428-005-1264-9.
- Eastwood, M. A. 2019. Dietary fibre, functions by modulating the entero-hepatic circulation. QJM: monthly Journal of the Association of Physicians 112 (11):833–4. doi: 10.1093/qjmed/hcz090.
- Edwards, C. 1990. Mechanisms of action on dietary fibre on small intestinal absorption and motility. In New developments in dietary fiber: Physiological, physicochemical, and analytical aspects, eds. I. Furda, and C. J. Brine, 95–104. New York: Springer.
- Edwards, C. H., P. Ryden, G. Mandalari, P. J. Butterworth, and P. R. Ellis. 2021. Structure-function studies of chickpea and durum wheat uncover mechanisms by which cell wall properties influence starch bioaccessibility. Nature Food 2 (2):118–26. doi: 10.1038/s43016-021-00230-y.
- Eggum, B. O. 1995. The influence of dietary fibre on protein digestion and utilization in monogastrics. Archiv Fur Tierernahrung 48 (1–2):89–95. doi: 10.1080/17450399509381831.
- Elangovan, S., R. Pathania, S. Ramachandran, S. Ananth, R. N. Padia, L. Lan, N. Singh, P. M. Martin, L. Hawthorn, P. D. Prasad, et al. 2014. The niacin/butyrate receptor GPR109A suppresses mammary tumorigenesis by inhibiting cell survival. Cancer Research 74 (4):1166–78. doi: 10.1158/0008-5472.CAN-13-1451.
- Espenshade, P. J., and A. L. Hughes. 2007. Regulation of sterol synthesis in eukaryotes. Annual Review of Genetics 41 (1):401–27. doi: 10.1146/annurev.genet.41.110306.130315.
- European Commission. 2020. Dietary fibre. https://ec.europa.eu/jrc/en/health-knowledge-gateway/promotion-prevention/nutrition/fibre.
- Evans, C. 2020. Dietary fibre and cardiovascular health: A review of current evidence and policy. The Proceedings of the Nutrition Society 79 (1):61–7. doi: 10.1017/S0029665119000673.
- Fabek, H., S. Messerschmidt, V. Brulport, and H. D. Goff. 2014. The effect of in vitro digestive processes on the viscosity of dietary fibres and their influence on glucose diffusion. Food Hydrocolloids 35:718–26. doi: 10.1016/j.foodhyd.2013.08.007.
- Ferguson, L. R., and P. J. Harris. 1996. Studies on the role of specific dietary fibres in protection against colorectal cancer. Mutation Research 350 (1):173–84. doi: 10.1016/0027-5107(95)00105-0.
- Fernandes, J., W. Su, S. Rahat-Rozenbloom, T. M. S. Wolever, and E. M. Comelli. 2014. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutrition & Diabetes 4 (6):e121–e121. doi: 10.1038/nutd.2014.23.
- Fetissov, S. O., J. Kopp, and T. Hökfelt. 2004. Distribution of NPY receptors in the hypothalamus. Neuropeptides 38 (4):175–88. doi: 10.1016/j.npep.2004.05.009.
- Fiszman, S., and P. Varela. 2013. The role of gums in satiety/satiation. A review. Food Hydrocolloids. 32 (1):147–54. doi: 10.1016/j.foodhyd.2012.12.010.
- Flint, H. J. 2011. Obesity and the gut microbiota. Journal of Clinical Gastroenterology 45:S128–S132. doi: 10.1097/MCG.0b013e31821f44c4.
- Flourie, B., N. Vidon, C. H. Florent, and J. J. Bernier. 1984. Effect of pectin on jejunal glucose absorption and unstirred layer thickness in normal man. Gut 25 (9):936–41. doi: 10.1136/gut.25.9.936.
- Gao, Z., J. Yin, J. Zhang, R. E. Ward, R. J. Martin, M. Lefevre, W. T. Cefalu, and J. Ye. 2009. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58 (7):1509–17. doi: 10.2337/db08-1637.
- Garattini, S., S. Cactia, T. Mennini, R. Samanin, S. Consolo, and H. Ladinsky. 1979. Biochemical pharmacology of the anorectic drug fenfluramine: A review. Current Medical Research and Opinion 6 (sup1):15–27. doi: 10.1185/03007997909117488.
- Gaskins, A. J., S. L. Mumford, C. Zhang, J. Wactawski-Wende, K. M. Hovey, B. W. Whitcomb, P. P. Howards, N. J. Perkins, E. Yeung, E. F. Schisterman, et al. 2009. Effect of daily fiber intake on reproductive function: The BioCycle Study. The American Journal of Clinical Nutrition 90 (4):1061–9.
- Gressier, M., and G. Frost. 2022. Minor changes in fibre intake in the UK population between 2008/2009 and 2016/2017. European Journal of Clinical Nutrition 76 (2):322–7. doi: 10.1038/s41430-021-00933-2.
- Gueugnon, C., F. Mougin, N. U. Nguyen, M. Bouhaddi, M. Nicolet-Guénat, and G. Dumoulin. 2012. Ghrelin and PYY levels in adolescents with severe obesity: Effects of weight loss induced by long-term exercise training and modified food habits. European Journal of Applied Physiology 112 (5):1797–805. doi: 10.1007/s00421-011-2154-2.
- Gunness, P., and M. J. Gidley. 2010. Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food & Function 1 (2):149–55.
- Haber, G. B., K. W. Heaton, D. Murphy, and L. F. Burroughs. 1977. depletion and disruption of dietary fibre: Effects on satiety, plasma-glucose, and serum-insulin. The Lancet 310 (8040):679–82. doi: 10.1016/S0140-6736(77)90494-9.
- Hamer, H. M., D. Jonkers, K. Venema, S. Vanhoutvin, F. J. Troost, and R.-J. Brummer. 2008. Review article: The role of butyrate on colonic function. Alimentary Pharmacology & Therapeutics 27 (2):104–19. doi: 10.1111/j.1365-2036.2007.03562.x.
- Hara, H., S. Haga, T. Kasai, and S. Kiriyama. 1998. Fermentation products of sugar-beet fiber by cecal bacteria lower plasma cholesterol concentration in rats. The Journal of Nutrition 128 (4):688–93.
- Hariharan, D., K. Vellanki, and H. Kramer. 2015. The western diet and chronic kidney disease. Current Hypertension Reports 17 (3):16. doi: 10.1007/s11906-014-0529-6.
- Harris, I. R., H. Höppner, W. Siefken, A. M. Farrell, and K. P. Wittern. 2000. Regulation of HMG-CoA synthase and HMG-CoA reductase by insulin and epidermal growth factor in HaCaT keratinocytes. The Journal of Investigative Dermatology 114 (1):83–7. doi: 10.1046/j.1523-1747.2000.00822.x.
- Harris, P. J., and L. R. Ferguson. 1993. Dietary fibre: Its composition and role in protection against colorectal cancer. Mutation Research 290 (1):97–110. doi: 10.1016/0027-5107(93)90037-G.
- Harvard. 2020. Harvard school of public health obesity causes. https://www.hsph.harvard.edu/obesity-prevention-source/obesity-causes/.
- Hassig, C. A., J. K. Tong, and S. L. Schreiber. 1997. Fiber-derived butyrate and the prevention of colon cancer. Chemistry & Biology 4 (11):783–9.
- Heaton, K. W. 1973. Food fibre as an obstacle to energy intake. The Lancet 302 (7843):1418–21. doi: 10.1016/S0140-6736(73)92806-7.
- Heisler, L. K., and D. D. Lam. 2017. An appetite for life: Brain regulation of hunger and satiety. Current Opinion in Pharmacology 37:100–6. doi: 10.1016/j.coph.2017.09.002.
- Hill, J. O. 2006. Understanding and addressing the epidemic of obesity: An energy balance perspective. Endocrine Reviews 27 (7):750–61. doi: 10.1210/er.2006-0032.
- Hogenkamp, P. S., and H. B. Schiöth. 2013. Effect of oral processing behaviour on food intake and satiety. Trends in Food Science & Technology 34 (1):67–75. doi: 10.1016/j.tifs.2013.08.010.
- Holland, C., P. Ryden, C. H. Edwards, and M. M.-L. Grundy. 2020. Plant cell walls: Impact on nutrient bioaccessibility and digestibility. Foods 9 (2):201. doi: 10.3390/foods9020201.
- Ichimura, A., S. Hasegawa, M. Kasubuchi, and I. Kimura. 2014. Free fatty acid receptors as therapeutic targets for the treatment of diabetes. Frontiers in Pharmacology 5 (236):236. doi: 10.3389/fphar.2014.00236.
- Istvan, E. S., and J. Deisenhofer. 2001. Structural mechanism for statin inhibition of HMG-CoA reductase. Science (New York, NY) 292 (5519):1160–4. doi: 10.1126/science.1059344.
- Jenkins, D. A. 1979. Dietary fibre, diabetes, and hyperlipidaemia: Progress and prospects. The Lancet 314 (8155):1287–90. doi: 10.1016/S0140-6736(79)92292-X.
- Jovanovski, E., N. Mazhar, A. Komishon, R. Khayyat, D. Li, S. Blanco Mejia, T. Khan, A. L. Jenkins, L. Smircic-Duvnjak, J. L. Sievenpiper, et al. 2021. Effect of viscous fiber supplementation on obesity indicators in individuals consuming calorie-restricted diets: A systematic review and meta-analysis of randomized controlled trials. European Journal of Nutrition 60 (1):101–12. doi: 10.1007/s00394-020-02224-1.
- Karaki, S.-I., H. Tazoe, H. Hayashi, H. Kashiwabara, K. Tooyama, Y. Suzuki, and A. Kuwahara. 2008. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. Journal of Molecular Histology 39 (2):135–42. doi: 10.1007/s10735-007-9145-y.
- Karin, M., Y. Cao, F. R. Greten, and Z.-W. Li. 2002. NF-kappaB in cancer: From innocent bystander to major culprit . Nature Reviews. Cancer 2 (4):301–10. doi: 10.1038/nrc780.
- Kelly, T., W. Yang, C.-S. Chen, K. Reynolds, and J. He. 2008. Global burden of obesity in 2005 and projections to 2030. International Journal of Obesity (2005) 32 (9):1431–7. doi: 10.1038/ijo.2008.102.
- Key, T. J., N. E. Allen, E. A. Spencer, and R. C. Travis. 2003. Nutrition and breast cancer. Breast (Edinburgh, Scotland) 12 (6):412–6. doi: 10.1016/s0960-9776(03)00145-0.
- Khanum, F., M. Siddalinga Swamy, K. R. Sudarshana Krishna, K. Santhanam, and K. R. Viswanathan. 2000. Dietary fiber content of commonly fresh and cooked vegetables consumed in India. Plant Foods for Human Nutrition (Dordrecht, Netherlands) 55 (3):207–18.
- Kim, Y. S., T. Unno, B. Y. Kim, and M. S. Park. 2020. Sex differences in gut microbiota. The World Journal of Men’s Health 38 (1):48–60. doi: 10.5534/wjmh.190009.
- Kimura, I., D. Inoue, K. Hirano, and G. Tsujimoto. 2014. The SCFA receptor GPR43 and energy metabolism. Frontiers in Endocrinology 5:85. doi: 10.3389/fendo.2014.00085.
- Klassen, L., X. Xing, J. P. Tingley, K. E. Low, M. L. King, G. Reintjes, and D. W. Abbott. 2021. Approaches to investigate selective dietary polysaccharide utilization by human gut microbiota at a functional level. Frontiers in Microbiology 12:632684. doi: 10.3389/fmicb.2021.632684.
- Kritchevsky, D. 1987. Dietary fibre and lipid metabolism in animals. Scandinavian Journal of Gastroenterology. Supplement 129 (sup129):213–7. doi: 10.3109/00365528709095887.
- Krzysztoszek, J., I. Laudańska-Krzemińska, and M. Bronikowski. 2019. Assessment of epidemiological obesity among adults in EU countries. Annals of Agricultural and Environmental Medicine: AAEM 26 (2):341–9. doi: 10.26444/aaem/97226.
- Larhammar, D. 1996. Structural diversity of receptors for neuropeptide Y, peptide YY and pancreatic polypeptide. Regulatory Peptides 65 (3):165–74. doi: 10.1016/0167-0115(96)00110-3.
- Lattimer, J. M., and M. D. Haub. 2010. Effects of dietary fiber and its components on metabolic health. Nutrients 2 (12):1266–89. doi: 10.3390/nu2121266.
- Le Poul, E., C. Loison, S. Struyf, J.-Y. Springael, V. Lannoy, M.-E. Decobecq, S. Brezillon, V. Dupriez, G. Vassart, J. Van Damme, et al. 2003. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. The Journal of Biological Chemistry 278 (28):25481–9. doi: 10.1074/jbc.M301403200.
- le Roux, C. W., R. L. Batterham, S. J. B. Aylwin, M. Patterson, C. M. Borg, K. J. Wynne, A. Kent, R. P. Vincent, J. Gardiner, M. A. Ghatei, et al. 2006. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 147 (1):3–8. doi: 10.1210/en.2005-0972.
- Lee, H.-R., K.-A. Hwang, M.-A. Park, B.-R. Yi, E.-B. Jeung, and K.-C. Choi. 2012. Treatment with bisphenol A and methoxychlor results in the growth of human breast cancer cells and alteration of the expression of cell cycle-related genes, cyclin D1 and p21, via an estrogen receptor-dependent signaling pathway. International Journal of Molecular Medicine 29 (5):883–90.
- Lee, J. 2020. The obesity pandemic and the search for solutions. Journal of Medicinal Food 23 (3):205. doi: 10.1089/jmf.2020.29006.lee.
- Ley, R. E., P. J. Turnbaugh, S. Klein, and J. I. Gordon. 2006. Human gut microbes associated with obesity. Nature 444 (7122):1022–3.
- Li, J., N. Zhang, L. Hu, Z. Li, R. Li, C. Li, and S. Wang. 2011. Improvement in chewing activity reduces energy intake in one meal and modulates plasma gut hormone concentrations in obese and lean young Chinese men1–3. The American Journal of Clinical Nutrition 94 (3):709–16. doi: 10.3945/ajcn.111.015164.
- Li, K.-K., P.-J. Tian, S.-D. Wang, P. Lei, L. Qu, J.-P. Huang, Y.-J. Shan, and B.-L. Li. 2017. Targeting gut microbiota: Lactobacillus alleviated type 2 diabetes via inhibiting LPS secretion and activating GPR43 pathway. Journal of Functional Foods 38:561–70. doi: 10.1016/j.jff.2017.09.049.
- Li, Q., T. R. Holford, Y. Zhang, P. Boyle, S. T. Mayne, M. Dai, and T. Zheng. 2013. Dietary fiber intake and risk of breast cancer by menopausal and estrogen receptor status. European Journal of Nutrition 52 (1):217–23. doi: 10.1007/s00394-012-0305-9.
- Li, S., A. Zong, R. An, H. Wang, L. Liu, J. Liu, X. Guo, Z. Xu, J. Wang, D. Li, et al. 2021. Effects of whole grain intake on glycemic traits: A systematic review and meta-analysis of randomized controlled trials. Critical Reviews in Food Science and Nutrition 1–20. doi: 10.1080/10408398.2021.2001429.
- Lin, Y., I. Huybrechts, S. Vandevijvere, S. Bolca, W. De Keyzer, S. De Vriese, A. Polet, M. De Neve, H. Van Oyen, J. Van Camp, et al. 2011. Fibre intake among the Belgian population by sex–age and sex–education groups and its association with BMI and waist circumference. British Journal of Nutrition 105 (11):1692–703. doi: 10.1017/S0007114510005088.
- Ludwig, D. S. 1999. Dietary fiber, weight gain, and cardiovascular disease risk factors in young adults. JAMA 282 (16):1539–46. doi: 10.1001/jama.282.16.1539.
- Lunn, J., and J. L. Buttriss. 2007. Carbohydrates and dietary fibre. Nutrition Bulletin 32 (1):21–64. doi: 10.1111/j.1467-3010.2007.00616.x.
- Macfarlane, S., and G. T. Macfarlane. 2003. Regulation of short-chain fatty acid production. The Proceedings of the Nutrition Society 62 (1):67–72. doi: 10.1079/PNS2002207.
- Mandaliya, D., S. Patel, and S. Seshadri. 2018. Fiber in our diet and its role in health and disease. In Functional food and human health, eds. V. Rani, and U. C. S. Yadav, 247–55. Singapore: Springer.
- Manzel, A., D. N. Muller, D. A. Hafler, S. E. Erdman, R. A. Linker, and M. Kleinewietfeld. 2014. Role of “western diet” in inflammatory autoimmune diseases. Current Allergy and Asthma Reports 14 (1):404. doi: 10.1007/s11882-013-0404-6.
- Mariat, D., O. Firmesse, F. Levenez, V. Guimarăes, H. Sokol, J. Doré, G. Corthier, and J.-P. Furet. 2009. The firmicutes/bacteroidetes ratio of the human microbiota changes with age. BMC Microbiology 9 (1):123. doi: 10.1186/1471-2180-9-123.
- Martínez-González, M. A., E. Fernández-Jarne, E. Martínez-Losa, M. Prado-Santamaría, C. Brugarolas-Brufau, and M. Serrano-Martinez. 2002. Role of fibre and fruit in the Mediterranean diet to protect against myocardial infarction: A case-control study in Spain. European Journal of Clinical Nutrition 56 (8):715–22. doi: 10.1038/sj.ejcn.1601382.
- Masrul, M., and R. D. Nindrea. 2019. Dietary fibre protective against colorectal cancer patients in Asia: A meta-analysis. Open Access Macedonian Journal of Medical Sciences 7 (10):1723–7. doi: 10.3889/oamjms.2019.265.
- McNeil, N. I., and D. S. Rampton. 1981. Is the rectum usually empty?—A quantitative study in subjects with and without diarrhea. Diseases of the Colon and Rectum 24 (8):596–9.
- Meier, J. J. 2012. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nature Reviews. Endocrinology 8 (12):728–42. doi: 10.1038/nrendo.2012.140.
- Merriam-Webster. 2020a. Microbiome. https://www.merriam-webster.com/dictionary/microbiome
- Merriam-Webster. 2020b. Microbiota. https://www.merriam-webster.com/dictionary/microbiota,
- Milagro, F. I., M. J. Moreno-Alaga, and J. A. Martinez. 2020. Nutrients, obesity and gene expression. Chapter 58. In Principles of nutrigenetics and nutrigenomics, ed. R. D. Caterina, J. Alfredo Martinez, and M. Kohlmeier, 431–40. London, UK: Academic Press.
- Miquel-Kergoat, S., V. Azais-Braesco, B. Burton-Freeman, and M. M. Hetherington. 2015. Effects of chewing on appetite, food intake and gut hormones: A systematic review and meta-analysis. Physiology & Behavior 151:88–96. doi: 10.1016/j.physbeh.2015.07.017.
- Montonen, J., P. Knekt, R. Järvinen, A. Aromaa, and A. Reunanen. 2003. Whole-grain and fiber intake and the incidence of type 2 diabetes. The American Journal of Clinical Nutrition 77 (3):622–9. doi: 10.1093/ajcn/77.3.622.
- Morell, P., S. M. Fiszman, P. Varela, and I. Hernando. 2014. Hydrocolloids for enhancing satiety: Relating oral digestion to rheology, structure and sensory perception. Food Hydrocolloids. 41:343–53. doi: 10.1016/j.foodhyd.2014.04.038.
- Müller, T. D., B. Finan, S. R. Bloom, D. D’Alessio, D. J. Drucker, P. R. Flatt, A. Fritsche, F. Gribble, H. J. Grill, J. F. Habener, et al. 2019. Glucagon-like peptide 1 (GLP-1). Molecular Metabolism 30:72–130. doi: 10.1016/j.molmet.2019.09.010.
- Murakami, K., S. Sasaki, H. Okubo, Y. Takahashi, Y. Hosoi, and M. Itabashi. 2007. Dietary fiber intake, dietary glycemic index and load, and body mass index: A cross-sectional study of 3931 Japanese women aged 18-20 years. European Journal of Clinical Nutrition 61 (8):986–95. doi: 10.1038/sj.ejcn.1602610.
- Musgrove, E. A., C. E. Caldon, J. Barraclough, A. Stone, and R. L. Sutherland. 2011. Cyclin D as a therapeutic target in cancer. Nature Reviews. Cancer 11 (8):558–72. doi: 10.1038/nrc3090.
- Nagengast, F., T. T. Ung, N. H. Kim, and Y. Do Jung. 1995. Role of bile acids in colorectal carcinogenesis. World Journal of Clinical Cases 6 (13):577–88.
- Nakajima, S., and M. Kitamura. 2013. Bidirectional regulation of NF-κB by reactive oxygen species: A role of unfolded protein response. Free Radical Biology & Medicine 65:162–74. doi: 10.1016/j.freeradbiomed.2013.06.020.
- Nakashima, A., K. Yamada, O. Iwata, R. Sugimoto, K. Atsuji, T. Ogawa, N. Ishibashi-Ohgo, and K. Suzuki. 2018. β-glucan in foods and its physiological functions. Journal of Nutritional Science and Vitaminology 64 (1):8–17. doi: 10.3177/jnsv.64.8.
- National Cancer Institute. 2017. Obesity and cancer. https://www.cancer.gov/about-cancer/causes-prevention/risk/obesity/obesity-fact-sheet.
- National Institute of Diabetes and Digestive and Kidney Diseases. 2015. Health risks of being overweight. https://www.niddk.nih.gov/health-information/weight-management/health-risks-overweight.
- Naumann, S., U. Schweiggert-Weisz, J. Eglmeier, D. Haller, and P. Eisner. 2019. In vitro interactions of dietary fibre enriched food ingredients with primary and secondary bile acids. Nutrients 11 (6):1424. doi: 10.3390/nu11061424.
- Nguyen, T. T., T. T. Ung, N. H. Kim, and Y. D. Jung. 2018. Role of bile acids in colon carcinogenesis. World Journal of Clinical Cases 6 (13):577–88. doi: 10.12998/wjcc.v6.i13.577.
- NHS. 2019. Obesity. https://www.nhs.uk/conditions/obesity/.
- NICE. 2015. Preventing excess weight gain. https://www.nice.org.uk/guidance/ng7.
- Nyberg, S. T., G. D. Batty, J. Pentti, M. Virtanen, L. Alfredsson, E. I. Fransson, M. Goldberg, K. Heikkilä, M. Jokela, A. Knutsson, et al. 2018. Obesity and loss of disease-free years owing to major non-communicable diseases: A multicohort study. The Lancet Public Health 3 (10):e490–e497. doi: 10.1016/S2468-2667(18)30139-7.
- O’Grady, J., E. M. O’Connor, and F. Shanahan. 2019. Review article: Dietary fibre in the era of microbiome science. Alimentary Pharmacology & Therapeutics 49 (5):506–15. doi: 10.1111/apt.15129.
- Ofei, F. 2005. Obesity - A preventable disease. Ghana Medical Journal 39 (3):98–101.
- Oldways Whole Grains Council. 2020. Whole grains A to Z. https://wholegrainscouncil.org/whole-grains-101/whole-grains-z.
- Ooi, L.-G., and M.-T. Liong. 2010. Cholesterol-lowering effects of probiotics and prebiotics: A review of in vivo and in vitro findings. International Journal of Molecular Sciences 11 (6):2499–522. doi: 10.3390/ijms11062499.
- Ou, S., K. Kwok, Y. Li, and L. Fu. 2001. In vitro study of possible role of dietary fiber in lowering postprandial serum glucose. Journal of Agricultural and Food Chemistry 49 (2):1026–9. doi: 10.1021/jf000574n.
- Park, M. H., and J. T. Hong. 2016. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells 5 (2):15. doi: 10.3390/cells5020015.
- Park, S., J. Kang, S. Choi, H. Park, E. Hwang, Y. Kang, A. Kim, W. Holzapfel, and Y. Ji. 2018. Cholesterol-lowering effect of Lactobacillus rhamnosus BFE5264 and its influence on the gut microbiome and propionate level in a murine model. Plos One 13 (8):e0203150. doi: 10.1371/journal.pone.0203150.
- Pasman, W. J., W. H. Saris, M. A. Wauters, and M. S. Westerterp-Plantenga. 1997. Effect of one week of fibre supplementation on hunger and satiety ratings and energy intake. Appetite 29 (1):77–87. doi: 10.1006/appe.1997.0091.
- Pirola, L., A. M. Johnston, and E. Van Obberghen. 2004. Modulation of insulin action. Diabetologia 47 (2):170–84. doi: 10.1007/s00125-003-1313-3.
- Place, R. F., E. J. Noonan, and C. Giardina. 2005. HDAC inhibition prevents NF-kappa B activation by suppressing proteasome activity: down-regulation of proteasome subunit expression stabilizes I kappa B alpha. Biochemical Pharmacology 70 (3):394–406. doi: 10.1016/j.bcp.2005.04.030.
- Poirier, P., T. D. Giles, G. A. Bray, Y. Hong, J. S. Stern, F. X. Pi-Sunyer, and R. H. Eckel. 2006. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss. Arteriosclerosis, Thrombosis, and Vascular Biology 26 (5):968–76. doi: 10.1161/01.ATV.0000216787.85457.f3.
- Post, R. E., A. G. Mainous, D. E. King, and K. N. Simpson. 2012. Dietary fiber for the treatment of type 2 diabetes mellitus: A meta-analysis. Journal of the American Board of Family Medicine: JABFM 25 (1):16–23. doi: 10.3122/jabfm.2012.01.110148.
- Public Health England. 2016. Government dietary recommendations. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/618167/government_dietary_recommendations.pdf.
- Public Health England. 2018. National diet and nutrition survey. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/699241/NDNS_results_years_7_and_8.pdf.
- Rafieian-Kopaei, M., M. Setorki, M. Doudi, A. Baradaran, and H. Nasri. 2014. Atherosclerosis: Process, indicators, risk factors and new hopes. International Journal of Preventive Medicine 5 (8):927–46.
- Rayasam, G. V., V. K. Tulasi, J. A. Davis, and V. S. Bansal. 2007. Fatty acid receptors as new therapeutic targets for diabetes. Expert Opinion on Therapeutic Targets 11 (5):661–71. doi: 10.1517/14728222.11.5.661.
- Ridley, B. L., M. A. O’Neill, and D. Mohnen. 2001. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57 (6):929–67. doi: 10.1016/S0031-9422(01)00113-3.
- Rijnaarts, I., N. de Roos, E. G. Zoetendal, N. de Wit, and B. J. M. Witteman. 2021. Development and validation of the FiberScreen: A short questionnaire to screen fibre intake in adults. Journal of Human Nutrition and Dietetics: The Official Journal of the British Dietetic Association 34 (6):969–80. doi: 10.1111/jhn.12941.
- Ríos-Covián, D., P. Ruas-Madiedo, A. Margolles, M. Gueimonde, C. G. de Los Reyes-Gavilán, and N. Salazar. 2016. Intestinal short chain fatty acids and their link with diet and human health. Frontiers in Microbiology 7:185. doi: 10.3389/fmicb.2016.00185.
- Roberfroid, M. 1993. Dietary fiber, inulin, and oligofructose: A review comparing their physiological effects. Critical Reviews in Food Science and Nutrition 33 (2):103–48. doi: 10.1080/10408399309527616.
- Robertson, M. D., J. M. Currie, L. M. Morgan, D. P. Jewell, and K. N. Frayn. 2003. Prior short-term consumption of resistant starch enhances postprandial insulin sensitivity in healthy subjects. Diabetologia 46 (5):659–65. doi: 10.1007/s00125-003-1081-0.
- Rock, C. L., S. W. Flatt, C. A. Thomson, M. L. Stefanick, V. A. Newman, L. A. Jones, L. Natarajan, C. Ritenbaugh, K. A. Hollenbach, J. P. Pierce, et al. 2004. Effects of a high-fiber, low-fat diet intervention on serum concentrations of reproductive steroid hormones in women with a history of breast cancer. Journal of Clinical Oncology 22 (12):2379–87. doi: 10.1200/JCO.2004.09.025.
- Rodwell, V. W. 1976. Regulation of HMG-CoA reductase. In Advances in lipid research, eds. R. Paoletti, D. and Kritchevsky, 1–74. London, UK: Elsevier.
- Röhrl, C., and H. Stangl. 2018. Cholesterol metabolism-physiological regulation and pathophysiological deregulation by the endoplasmic reticulum. Wiener Medizinische Wochenschrift (1946) 168 (11–12):280–5. doi: 10.1007/s10354-018-0626-2.
- Rose, D. P. 1992. Dietary fiber, phytoestrogens, and breast cancer. Nutrition (Burbank, Los Angeles County, CA) 8 (1):47–51.
- Russo, J., and I. H. Russo. 2006. The role of estrogen in the initiation of breast cancer. The Journal of Steroid Biochemistry and Molecular Biology 102 (1–5):89–96. doi: 10.1016/j.jsbmb.2006.09.004.
- Scheppach, W., H. P. Bartram, and F. Richter. 1995. Role of short-chain fatty acids in the prevention of colorectal cancer. European Journal of Cancer 31 (7–8):1077–80. doi: 10.1016/0959-8049(95)00165-F.
- Schottelius, A., and A. S. Baldwin. Jr. 1999. A role for transcription factor NF-kappa B in intestinal inflammation . International Journal of Colorectal Disease 14 (1):18–28. doi: 10.1007/s003840050178.
- Schwiertz, A., D. Taras, K. Schäfer, S. Beijer, N. A. Bos, C. Donus, and P. D. Hardt. 2010. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring, MD) 18 (1):190–5. doi: 10.1038/oby.2009.167.
- Singh, J. E., A.-K. Illner, K. Dokova, N. Usheva, T. Kostadinova, and K. Aleksandrova. 2020. Mapping the global evidence on nutrition transition: A scoping review protocol. BMJ Open 10 (6):e034730. doi: 10.1136/bmjopen-2019-034730.
- Singh, N., A. Gurav, S. Sivaprakasam, E. Brady, R. Padia, H. Shi, M. Thangaraju, P. D. Prasad, S. Manicassamy, D. H. Munn, et al. 2014. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40 (1):128–39. doi: 10.1016/j.immuni.2013.12.007.
- Slavin, J., and H. Green. 2007. Dietary fibre and satiety. Nutrition Bulletin 32 (s1):32–42. doi: 10.1111/j.1467-3010.2007.00603.x.
- Sloan, K. J., and J. W. McRorie. 2017. Dietary fiber: All fibers are not alike. In Nutrition guide for physicians and related healthcare professionals, N. J. Temple, 229–39. New York: Springer International Publishing.
- Song, Y.-J., M. Sawamura, K. Ikeda, S. Igawa, and Y. Yamori. 2000. Soluble dietary fibre improves insulin sensitivity by increasing muscle glut-4 content in stroke-prone spontaneously hypertensive rats. Clinical and Experimental Pharmacology and Physiology 27 (1–2):41–5. doi: 10.1046/j.1440-1681.2000.03198.x.
- Spiller, G. A., R. J. Amen, and D. Kritchevsky. 1975. Dietary fiber in human nutrition. CRC Critical Reviews in Food Science and Nutrition 7 (1):39–70. doi: 10.1080/10408397509527201.
- Stanaway, J. D., A. Afshin, E. Gakidou, S. S. Lim, D. Abate, K. H. Abate, C. Abbafati, N. Abbasi, H. Abbastabar, F. Abd-Allah, et al. 2018. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet 392 (10159):1923–94. doi: 10.1016/S0140-6736(18)32225-6.
- Stephen, A. M., M. M.-J. Champ, S. J. Cloran, M. Fleith, L. van Lieshout, H. Mejborn, and V. J. Burley. 2017. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutrition Research Reviews 30 (2):149–90. doi: 10.1017/S095442241700004X.
- Stoll, B. A. 1996. Can supplementary dietary fibre suppress breast cancer growth? British Journal of Cancer 73 (5):557–9. doi: 10.1038/bjc.1996.97.
- Story, J. A., and S. L. Lord. 1987. Bile salts: In vitro studies with fibre components. Scandinavian Journal of Gastroenterology. Supplement 129 (sup129):174–80. doi: 10.3109/00365528709095880.
- Sui, M., H. Zhang, and W. Fan. 2011. The role of estrogen and estrogen receptors in chemoresistance. Current Medicinal Chemistry 18 (30):4674–83. doi: 10.2174/092986711797379348.
- Sung, M.-K., J.-Y. Yeon, S.-Y. Park, J. H. Y. Park, and M.-S. Choi. 2011. Obesity-induced metabolic stresses in breast and colon cancer. Annals of the New York Academy of Sciences 1229:61–8. doi: 10.1111/j.1749-6632.2011.06094.x.
- Sutherland, E. W., and G. A. Robison. 1969. The role of cyclic AMP in the control of carbohydrate metabolism. Diabetes 18 (12):797–819. doi: 10.2337/diab.18.12.797.
- Suzuki, K., K. A. Simpson, J. S. Minnion, J. C. Shillito, and S. R. Bloom. 2010. The role of gut hormones and the hypothalamus in appetite regulation. Endocrine Journal 57 (5):359–72. doi: 10.1507/endocrj.k10e-077.
- Swaminath, G. 2008. Fatty acid binding receptors and their physiological role in type 2 diabetes. Archiv Der Pharmazie 341 (12):753–61. doi: 10.1002/ardp.200800096.
- Swinburn, B. A., G. Sacks, K. D. Hall, K. McPherson, D. T. Finegood, M. L. Moodie, and S. L. Gortmaker. 2011. The global obesity pandemic: Shaped by global drivers and local environments. The Lancet 378 (9793):804–14. doi: 10.1016/S0140-6736(11)60813-1.
- Tan, J, C. McKenzie, M. Potamitis, A. N. Thorburn, C. R. Mackay, and L. Macia. 2014. The role of short-chain fatty acids in health and disease. Chapter 3. In Advances in immunology, F. W. Alt, 91–119. London, UK: Academic Press.
- Thangaraju, M., G. A. Cresci, K. Liu, S. Ananth, J. P. Gnanaprakasam, D. D. Browning, J. D. Mellinger, S. B. Smith, G. J. Digby, N. A. Lambert, et al. 2009. GPR109A Is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Research 69 (7):2826–32. doi: 10.1158/0008-5472.CAN-08-4466.
- The BMJ. Chapter 4. Measurement error and bias. https://www.bmj.com/about-bmj/resources-readers/publications/epidemiology-uninitiated/4-measurement-error-and-bias.
- The InterAct Consortium. 2015. Dietary fibre and incidence of type 2 diabetes in eight European countries: The EPIC-InterAct Study and a meta-analysis of prospective studies. Diabetologia 58 (7):1394–408.
- Thomas, M. P., and B. Potter. 2013. The structural biology of oestrogen metabolism. The Journal of Steroid Biochemistry and Molecular Biology 137:27–49. doi: 10.1016/j.jsbmb.2012.12.014.
- Thorpe, R. J., Jr., and K. F. Ferraro. 2004. Aging, obesity, and mortality: Misplaced concern about obese older people? Research on Aging 26 (1):108–29. doi: 10.1177/0164027503258738.
- Threapleton, D. E., D. C. Greenwood, C. E. L. Evans, C. L. Cleghorn, C. Nykjaer, C. Woodhead, J. E. Cade, C. P. Gale, and V. J. Burley. 2013. Dietary fibre intake and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ (Clinical Research ed.) 347:f6879. doi: 10.1136/bmj.f6879.
- Tiwari, A. 2010. GPR43: An emerging target for the potential treatment of type 2 diabetes, obesity and insulin resistance. Current Opinion in Investigational Drugs (London, England: 2000) 11 (4):385–93.
- Topping, D. L. 1996. Short-chain fatty acids produced by intestinal bacteria. Asia Pacific Journal of Clinical Nutrition 5 (1):15–9.
- Topping, D. L., D. Oakenfull, R. P. Trimble, and R. J. Illman. 1988. A viscous fibre (methylcellulose) lowers blood glucose and plasma triacylglycerols and increases liver glycogen independently of volatile fatty acid production in the rat. The British Journal of Nutrition 59 (1):21–30. doi: 10.1079/bjn19880006.
- Travis, R. C., and T. J. Key. 2003. Oestrogen exposure and breast cancer risk. Breast Cancer Research: BCR 5 (5):239–47. doi: 10.1186/bcr628.
- Turnbaugh, P. J., R. E. Ley, M. A. Mahowald, V. Magrini, E. R. Mardis, and J. I. Gordon. 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444 (7122):1027–31.
- Turner, N. C., P. Neven, S. Loibl, and F. Andre. 2017. Advances in the treatment of advanced oestrogen-receptor-positive breast cancer. The Lancet 389 (10087):2403–14. doi: 10.1016/S0140-6736(16)32419-9.
- Ueno, H., H. Yamaguchi, M. Mizuta, and M. Nakazato. 2008. The role of PYY in feeding regulation. Regulatory Peptides 145 (1-3):12–6. doi: 10.1016/j.regpep.2007.09.011.
- Ulven, T. 2012. Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Frontiers in Endocrinology 3:111. doi: 10.3389/fendo.2012.00111.
- Upson, K., A. J. De Roos, M. L. Thompson, S. Sathyanarayana, D. Scholes, D. B. Barr, and V. L. Holt. 2013. Organochlorine pesticides and risk of endometriosis: Findings from a population-based case-control study. Environmental Health Perspectives 121 (11–12):1319–24. doi: 10.1289/ehp.1306648.
- van de Vijver, L. P. L., L. M. C. van den Bosch, P. A. van den Brandt, and R. A. Goldbohm. 2009. Whole-grain consumption, dietary fibre intake and body mass index in the Netherlands cohort study. European Journal of Clinical Nutrition 63 (1):31–8. doi: 10.1038/sj.ejcn.1602895.
- van Duursen, M. 2017. Modulation of estrogen synthesis and metabolism by phytoestrogens in vitro and the implications for women’s health. Toxicology Research 6 (6):772–94. doi: 10.1039/c7tx00184c.
- Viardot, A., L. K. Heilbronn, H. Herzog, S. Gregersen, and L. V. Campbell. 2008. Abnormal postprandial PYY response in insulin sensitive nondiabetic subjects with a strong family history of type 2 diabetes. International Journal of Obesity 32 (6):943–8. doi: 10.1038/ijo.2008.24.
- Wakai, K., C. Date, M. Fukui, K. Tamakoshi, Y. Watanabe, N. Hayakawa, M. Kojima, M. Kawado, K. Suzuki, S. Hashimoto, et al. 2007. Dietary fiber and risk of colorectal cancer in the Japan Collaborative Cohort Study. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 16 (4):668–75.
- Wanders, A. J., M. C. Jonathan, J. J. G. C. van den Borne, M. Mars, H. A. Schols, E. J. M. Feskens, and C. de Graaf. 2013. The effects of bulking, viscous and gel-forming dietary fibres on satiation. British Journal of Nutrition 109 (7):1330–7. doi: 10.1017/S0007114512003145.
- Wang, A., Z. Gu, B. Heid, R. M. Akers, and H. Jiang. 2009. Identification and characterization of the bovine G protein-coupled receptor GPR41 and GPR43 genes1. Journal of Dairy Science 92 (6):2696–705. doi: 10.3168/jds.2009-2037.
- Wang, P.-Y., J.-C. Fang, Z.-H. Gao, C. Zhang, and S.-Y. Xie. 2016. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: A meta-analysis. Journal of Diabetes Investigation 7 (1):56–69. doi: 10.1111/jdi.12376.
- Waris, G., and H. Ahsan. 2006. Reactive oxygen species: Role in the development of cancer and various chronic conditions. Journal of Carcinogenesis 5:14. doi: 10.1186/1477-3163-5-14.
- Wehrli, F., P. E. Taneri, A. Bano, L. Bally, L. C. Blekkenhorst, W. Bussler, B. Metzger, B. Minder, M. Glisic, T. Muka, et al. 2021. Oat intake and risk of type 2 diabetes, cardiovascular disease and all-cause mortality: A systematic review and meta-analysis. Nutrients 13 (8):2560. doi: 10.3390/nu13082560.
- Wolever, T. M., P. J. Spadafora, S. C. Cunnane, and P. B. Pencharz. 1995. Propionate inhibits incorporation of colonic [1,2-13C]acetate into plasma lipids in humans. The American Journal of Clinical Nutrition 61 (6):1241–7. doi: 10.1093/ajcn/61.6.1241.
- Wolever, T. M., P. Spadafora, and H. Eshuis. 1991. Interaction between colonic acetate and propionate in humans. The American Journal of Clinical Nutrition 53 (3):681–7. doi: 10.1093/ajcn/53.3.681.
- Wong, J., R. de Souza, C. W. C. Kendall, A. Emam, and D. J. A. Jenkins. 2006. Colonic health: Fermentation and short chain fatty acids. Journal of Clinical Gastroenterology 40 (3):235–43.
- World Health Organization (WHO). 2019. Diet, nutrition and the prevention of chronic diseases report of the joint WHO/FAO expert consultation. https://www.who.int/dietphysicalactivity/publications/trs916/summary/en/.
- World Health Organization (WHO). 2020. Obesity and overweight. https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight.
- World Obesity. 2019. Prevalence of obesity. https://www.worldobesity.org/about/about-obesity/prevalence-of-obesity.
- Wright, R. S., J. W. Anderson, and S. R. Bridges. 1990. Propionate inhibits hepatocyte lipid synthesis. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, NY) 195 (1):26–9. doi: 10.3181/00379727-195-43113.
- Yoon, K., and N. Kim. 2018. The effect of microbiota on colon carcinogenesis. Journal of Cancer Prevention 23 (3):117–25. doi: 10.15430/JCP.2018.23.3.117.
- Yu, K, et al. 2014. The impact of soluble dietary fibre on gastric emptying, postprandial blood glucose and insulin in patients with type 2 diabetes. Asia Pacific Journal of Clinical Nutrition 23 (2):210.
- Yulyaningsih, E., L. Zhang, H. Herzog, and A. Sainsbury. 2011. NPY receptors as potential targets for anti-obesity drug development. British Journal of Pharmacology 163 (6):1170–202. doi: 10.1111/j.1476-5381.2011.01363.x.
- Zhang, C.-X., S. C. Ho, S.-Z. Cheng, Y.-M. Chen, J.-H. Fu, and F.-Y. Lin. 2011. Effect of dietary fiber intake on breast cancer risk according to estrogen and progesterone receptor status. European Journal of Clinical Nutrition 65 (8):929–36. doi: 10.1038/ejcn.2011.57.
- Zhang, Y., B. Sun, D. Feng, H. Hu, M. Chu, Q. Qu, J. T. Tarrasch, S. Li, T. Sun Kobilka, B. K. Kobilka, et al. 2017. Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature 546 (7657):248–53.